At the Kyoto climate conference in
December 1997, policymakers, climate experts, and industrial leaders came
together to seriously consider future global climate. Their goal: to
negotiate limits on the future release into the atmosphere of carbon dioxide
(CO2), the most plentiful greenhouse gas, in order to prevent
further human-driven climate change. The conferees took to heart the latest
scientific findings on global climate processes that have resulted, in large
part, from climate models.
![]()
Climate models are a major tool that scientists use to understand the complex web of climate mechanisms. However, important uncertainties remain. Scientists at Lawrence Livermore are actively seeking answers to the questions of how much climate is changing and how much CO2 is stored naturally in the atmosphere and oceans, ultimately helping to resolve the larger uncertainty: what effect human activities have on our environment.
![]() Climate modeling began in the 1960s,
when the National Oceanic and Atmospheric Administration initiated
development of general circulation models--complex mathematical descriptions
of the physics and dynamics of the atmosphere and oceans. Since then,
climate scientists here and elsewhere have been refining these models and
formulating an entire hierarchy of other models to elucidate global climate.
Their ultimate goal is a virtual global climate system, a model that will
allow them to confidently predict future global and regional climate.
Climate modeling began in the 1960s,
when the National Oceanic and Atmospheric Administration initiated
development of general circulation models--complex mathematical descriptions
of the physics and dynamics of the atmosphere and oceans. Since then,
climate scientists here and elsewhere have been refining these models and
formulating an entire hierarchy of other models to elucidate global climate.
Their ultimate goal is a virtual global climate system, a model that will
allow them to confidently predict future global and regional climate.
![]() Development of such a model is far from
complete. But with global climate change at the forefront of public
attention and policymaking agendas, climate scientists must provide input to
policymakers based on today's imperfect models while pressing forward to
improve them. Indeed, they are working under some urgency, for the world now
appears ready to take action against global warming.
Development of such a model is far from
complete. But with global climate change at the forefront of public
attention and policymaking agendas, climate scientists must provide input to
policymakers based on today's imperfect models while pressing forward to
improve them. Indeed, they are working under some urgency, for the world now
appears ready to take action against global warming.
![]() One important question that models of
the carbon cycle answer is what the future atmospheric concentrations of CO2
may be. Carbon dioxide, a major greenhouse gas, is important to Earth's
energy balance and climate. Because CO2 concentrations have been
increasing rapidly, climatologists have pinpointed CO2 as a major
cause of the increase in global temperatures observed since 1860, when
complete temperature records began. It is no wonder, then, that the global
carbon cycle has become a vigorous area of study for the Climate System
Modeling Group in Lawrence Livermore's Earth and Environmental Sciences
Directorate. (See S&TR,
October 1996, for an overview of climate work in the Atmospheric
Sciences Division, much of which stems from Livermore's multidisciplinary
capabilities in scientific computation, nuclear testing, and atmospheric
science.) The group also collaborates with Department of Energy sponsors and
universities.
One important question that models of
the carbon cycle answer is what the future atmospheric concentrations of CO2
may be. Carbon dioxide, a major greenhouse gas, is important to Earth's
energy balance and climate. Because CO2 concentrations have been
increasing rapidly, climatologists have pinpointed CO2 as a major
cause of the increase in global temperatures observed since 1860, when
complete temperature records began. It is no wonder, then, that the global
carbon cycle has become a vigorous area of study for the Climate System
Modeling Group in Lawrence Livermore's Earth and Environmental Sciences
Directorate. (See S&TR,
October 1996, for an overview of climate work in the Atmospheric
Sciences Division, much of which stems from Livermore's multidisciplinary
capabilities in scientific computation, nuclear testing, and atmospheric
science.) The group also collaborates with Department of Energy sponsors and
universities.
Determining Carbon's Fate
![]() In nature, carbon is plentiful and
dynamic. Its natural cycle is thought to have been in a relatively steady
state for thousands of years until the industrial revolution. Then, humans
perturbed the carbon cycle by clearing forests and burning fossil fuels
(coal, oil, and natural gas), which increased the CO2 content of
the atmosphere. How has this anthropogenic change disrupted Earth's climate?
To find out, scientists are obtaining a better understanding of how the
climate system functions and tracking CO2 released into the
environment by humans.
In nature, carbon is plentiful and
dynamic. Its natural cycle is thought to have been in a relatively steady
state for thousands of years until the industrial revolution. Then, humans
perturbed the carbon cycle by clearing forests and burning fossil fuels
(coal, oil, and natural gas), which increased the CO2 content of
the atmosphere. How has this anthropogenic change disrupted Earth's climate?
To find out, scientists are obtaining a better understanding of how the
climate system functions and tracking CO2 released into the
environment by humans.
![]() Philip B. Duffy, group leader of the
Climate System Modeling Group, says that their work includes modeling how a
portion of anthropogenic CO2 remains in the atmosphere and how
the rest of it is taken up by the oceans and terrestrial ecosystems.
Tracking CO2 is neither simple nor easy: measurements of CO2
in the ocean and terrestrial biosphere are scarce; CO2 released
by humans is indistinguishable from naturally occurring CO2; and
the natural fluxes of carbon between ocean, atmosphere, and terrestrial
biosphere are poorly understood.
Philip B. Duffy, group leader of the
Climate System Modeling Group, says that their work includes modeling how a
portion of anthropogenic CO2 remains in the atmosphere and how
the rest of it is taken up by the oceans and terrestrial ecosystems.
Tracking CO2 is neither simple nor easy: measurements of CO2
in the ocean and terrestrial biosphere are scarce; CO2 released
by humans is indistinguishable from naturally occurring CO2; and
the natural fluxes of carbon between ocean, atmosphere, and terrestrial
biosphere are poorly understood.
![]() Lawrence Livermore modelers have been
formulating and testing a suite of models to reproduce carbon absorption,
transport, and storage processes. The models cannot possibly incorporate all
climate factors everywhere--even Livermore's advanced supercomputers cannot
provide that resolution--so modelers must select the most important climatic
factors and influences and represent them as well as possible.
Lawrence Livermore modelers have been
formulating and testing a suite of models to reproduce carbon absorption,
transport, and storage processes. The models cannot possibly incorporate all
climate factors everywhere--even Livermore's advanced supercomputers cannot
provide that resolution--so modelers must select the most important climatic
factors and influences and represent them as well as possible.
![]() The goal of the carbon cycle models is
to predict the future behavior of carbon in the climate system. The models
are tested by "hindcasting" the behavior of the carbon cycle from the
historical past to the present. If model simulations match past records and
observations, then model calculations predicting the future will merit
confidence.
The goal of the carbon cycle models is
to predict the future behavior of carbon in the climate system. The models
are tested by "hindcasting" the behavior of the carbon cycle from the
historical past to the present. If model simulations match past records and
observations, then model calculations predicting the future will merit
confidence.
The Ocean Carbon Cycle
![]() The Climate System Modeling Group's work
involves both terrestrial and ocean carbon cycles, with current emphasis on
the ocean. The ocean is an important regulator of climate as well as a major
absorber of excess CO2. Livermore modelers, having access to
powerful, next-generation computers from the Department of Energy's
Accelerated Strategic Computing Initiative, are able to formulate models
that represent ocean currents, eddies, and interactive processes with more
fidelity than previously possible. Their most recent ocean models reflect
the advance of climate simulation techniques and skills. The projects
described here demonstrate the approaches that modelers use to test
hypotheses about carbon cycle dynamics, understand process interactions, and
refine representations of the processes to derive accurate and useful
models.
The Climate System Modeling Group's work
involves both terrestrial and ocean carbon cycles, with current emphasis on
the ocean. The ocean is an important regulator of climate as well as a major
absorber of excess CO2. Livermore modelers, having access to
powerful, next-generation computers from the Department of Energy's
Accelerated Strategic Computing Initiative, are able to formulate models
that represent ocean currents, eddies, and interactive processes with more
fidelity than previously possible. Their most recent ocean models reflect
the advance of climate simulation techniques and skills. The projects
described here demonstrate the approaches that modelers use to test
hypotheses about carbon cycle dynamics, understand process interactions, and
refine representations of the processes to derive accurate and useful
models.
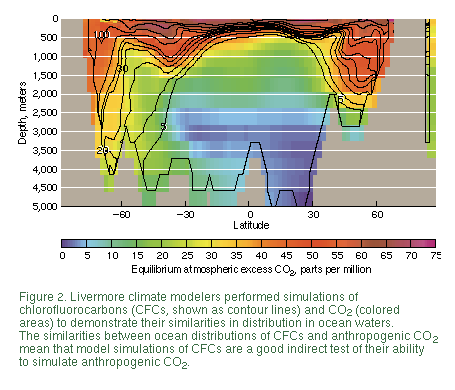
Improving Convection Models
![]() The ocean is a large "sink" for
anthropogenic CO2. A model that can accurately simulate how the
ocean absorbs anthropogenic CO2 is prerequisite to predicting
future atmospheric CO2 concentrations and rates of global
warming. But according to climate modelers Duffy and Ken Caldeira, current
ocean models may overpredict the amounts of CO2 that oceans
absorb. One reason is that models do not accurately represent convection,
the rapid vertical mixing of water that occurs when dense water overlies
less dense water. They saw the effects of that inaccuracy in models
involving sea-ice formation, which is a major cause of ocean convection.
The ocean is a large "sink" for
anthropogenic CO2. A model that can accurately simulate how the
ocean absorbs anthropogenic CO2 is prerequisite to predicting
future atmospheric CO2 concentrations and rates of global
warming. But according to climate modelers Duffy and Ken Caldeira, current
ocean models may overpredict the amounts of CO2 that oceans
absorb. One reason is that models do not accurately represent convection,
the rapid vertical mixing of water that occurs when dense water overlies
less dense water. They saw the effects of that inaccuracy in models
involving sea-ice formation, which is a major cause of ocean convection.
![]() When sea ice is forming, it expels salt
into surrounding surface water.
When sea ice is forming, it expels salt
into surrounding surface water.
The saltier, denser water triggers convection, sinking the surface water and
CO2 it contains into the depths of the ocean. Surface ocean
concentrations of CO2 are thus reduced, and more CO2
transfers from the air to the sea.
![]() In the real ocean, convection occurs in
horizontal regions that are meters or hundreds of meters across. Because
ocean models cannot accurately represent such small features, some
significant inaccuracies can arise in their simulations. Duffy and Caldeira
thought that the modeled transfer of CO2 from air to sea was
probably too great because the models simulated excessive convection. If the
instabilities resulting from expelled salt were treated more carefully,
model results might better represent reality.
In the real ocean, convection occurs in
horizontal regions that are meters or hundreds of meters across. Because
ocean models cannot accurately represent such small features, some
significant inaccuracies can arise in their simulations. Duffy and Caldeira
thought that the modeled transfer of CO2 from air to sea was
probably too great because the models simulated excessive convection. If the
instabilities resulting from expelled salt were treated more carefully,
model results might better represent reality.
![]() The two modelers tested their hypothesis
by performing a pair of simulations that compared the standard treatment of
convection to a "test" simulation in which the model's convection mechanism
was partially suppressed. In the standard "control" simulation, salt
released during sea-ice formation is placed in the model's top layer (which
is 25 meters thick), and the model's convection mechanism mixes it into the
rest of the ocean. Because the convection mechanism is clumsy, excessive
convection occurs. This in turn causes excessive ocean absorption of carbon.
The two modelers tested their hypothesis
by performing a pair of simulations that compared the standard treatment of
convection to a "test" simulation in which the model's convection mechanism
was partially suppressed. In the standard "control" simulation, salt
released during sea-ice formation is placed in the model's top layer (which
is 25 meters thick), and the model's convection mechanism mixes it into the
rest of the ocean. Because the convection mechanism is clumsy, excessive
convection occurs. This in turn causes excessive ocean absorption of carbon.
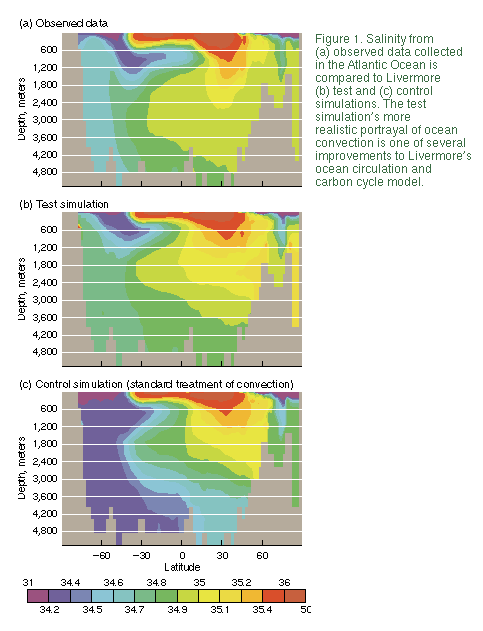
![]() In their test simulation, Duffy and
Caldeira dispersed expelled salt uniformly over a broader area--from the
surface down to a depth of 160 meters-- and suppressed the model's
convection mechanism (Figure 1). This simulation produced much more
realistic results for simulated convection, salinity, circulation, and
absorption of chlorofluorocarbons (CFCs).
In their test simulation, Duffy and
Caldeira dispersed expelled salt uniformly over a broader area--from the
surface down to a depth of 160 meters-- and suppressed the model's
convection mechanism (Figure 1). This simulation produced much more
realistic results for simulated convection, salinity, circulation, and
absorption of chlorofluorocarbons (CFCs).

 PHILIP B. DUFFY is a physicist at the
Laboratory, where he is group leader for the Climate System Modeling Group in
the Atmospheric Science Division. Duffy worked in strategic defense systems when
he joined the Laboratory in 1986. Prior to that, he received his Ph.D. and M.S.
in astrophysics in 1986 and 1981 from Stanford University and an A.B. in
astronomy and astrophysics in 1979 from Harvard University. Duffy has published
research on astronomy, atomic physics, and numerical modeling of ocean
circulation.
PHILIP B. DUFFY is a physicist at the
Laboratory, where he is group leader for the Climate System Modeling Group in
the Atmospheric Science Division. Duffy worked in strategic defense systems when
he joined the Laboratory in 1986. Prior to that, he received his Ph.D. and M.S.
in astrophysics in 1986 and 1981 from Stanford University and an A.B. in
astronomy and astrophysics in 1979 from Harvard University. Duffy has published
research on astronomy, atomic physics, and numerical modeling of ocean
circulation.  KENNETH G. CALDEIRA joined the Laboratory's
Atmospheric Chemistry Group as a physicist in 1993 and has been an environmental
scientist in the Climate System Modeling Group since 1995. He received his Ph.D.
and M.S. in atmospheric science from New York University in 1991 and 1988 and
his B.A. in philosophy from Rutgers University in 1978. He also served as a
postdoc at Pennsylvania State University's Earth System Science Center. Caldeira
has published many papers, for example, on climate stability of early Earth and
the global carbon cycle as it has been affected by human activity over millions
of years.
KENNETH G. CALDEIRA joined the Laboratory's
Atmospheric Chemistry Group as a physicist in 1993 and has been an environmental
scientist in the Climate System Modeling Group since 1995. He received his Ph.D.
and M.S. in atmospheric science from New York University in 1991 and 1988 and
his B.A. in philosophy from Rutgers University in 1978. He also served as a
postdoc at Pennsylvania State University's Earth System Science Center. Caldeira
has published many papers, for example, on climate stability of early Earth and
the global carbon cycle as it has been affected by human activity over millions
of years.