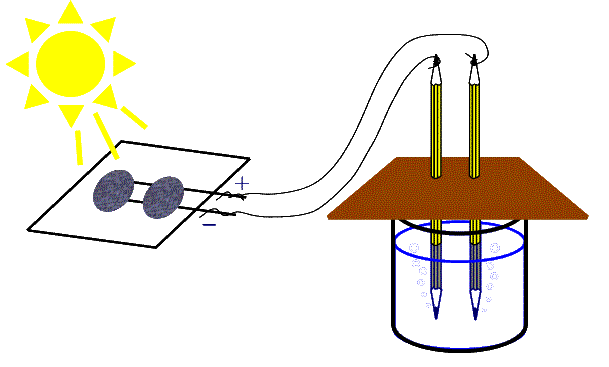

This project involves a fascinating experiment in electrochemistry that illustrates several important energy related processes, and provides an ideal context for discussion of several issues related to electricity generation.
As covered in the discussion section below, it is possible to use hydrogen as a fuel, that is, a way to store energy, for days when the Sun doesn't shine, or at night time, or for powered mobile devices such as cars.
The process by which we generate hydrogen (and oxygen) from water is called electrolysis. The word "lysis" means to dissolve or break apart, so the word "electrolysis" literally means to break something apart (in this case water) using electricity.
Electrolysis is very simple - all you have to do is arrange for electricity to pass through some water between to electrodes placed in the water, as shown in the diagram above. Its as simple as that! The principle of electrolysis was first formulated by Michael Faraday in 1820.
If the electricity used for electrolysis is generated from fossil fuels, then carbon dioxide would be emitted in support of our electrolysis process, and the advantage of using hydrogen as a fuel would be lost. But if the electricity is produced by solar cells, as we suggest in the diagram above, then there will be no pollutants released by our process.
Advanced students may want to study the efficiency of the electrolysis project. This can be done, under careful supervision (since you will be collecting hydrogen), in the following way:
Vtheoretical = (R I T t) / (F p z),
where R=8.314 Joule/(mol Kelvin), I = current in amps, T is the temperature in Kelvins (273 + Celsius temperature), t = time in seconds, F = Faraday's constant = 96485 Coulombs per mol, p = ambient pressure = about 1 x 105 pascals (one pascal = 1 Joule/meter3), z = number of "excess" electrons = 2 (for hydrogen, H2), 4 (if you're measuring oxygen production instead).
Efficiency (in %) = 100 x Vproduced / Vtheoretical .

The chemical equation for electrolysis is:
energy (electricity) + 2 H2O -> O2 + 2 H2 .
At the cathode (the negative electrode), there is a negative charge created by the battery. This means that there is an electrical pressure to push electrons into the water at this end. At the anode (the positive electrode), there is a positive charge, so that electrode would like to absorb electrons. But the water isn't a very good conductor. Instead, in order for there to be a flow of charge all the way around the circuit, water molecules near the cathode are split up into a positively charged hydrogen ion, which is symbolized as H+ in the diagram above (this is just the hydrogen atom without its electron, i.e. the nucleus of the hydrogen atom, which is just a single proton), and a negatively charged "hydroxide" ion, symbolized OH-:
H2O -> H+ + OH- .
You might have expected that H2O would break up into an H and an OH (the same atoms but with neutral charges) instead, but this doesn't happen because the oxygen atom more strongly attracts the electron from the H - it steals it (we say the oxygen atom is more "electronegative" than hydrogen). This theft allows the resulting hydroxide ion to have a completely filled outer shell, making it more stable.
But the H+, which is just a naked proton, is now free to pick up an electron (symbolized e-) from the cathode, which is trying hard to donate electrons, and become a regular, neutral hydrogen atom:
H+ + e- -> H
This hydrogen atom meets another hydrogen atom and forms a hydrogen gas molecule:
H + H -> H2,
and this molecule bubbles to the surface, and wa-la! We have hydrogen gas!
Meanwhile, the positive anode has caused the negatively charged hydroxide ion (OH-) to travel across the container to the anode. When it gets to the anode, the anode removes the extra electron that the hydroxide stole from the hydrogen atom earlier, and the hydroxide ion then recombines with three other hydroxide molecules to form 1 molecule of oxygen and 2 molecules of water:
4 OH- _> O2 + 2 H2O + 4e-
The oxygen molecule is very stable, and bubbles to the surface.
In this way, a closed circuit is created, involving negatively charged particles - electrons in the wire, hydroxide ions in the water. The energy delivered by the battery is stored by the production of hydrogen.
Water is perhaps the most important substance to life on Earth. It is a simple compound made from the two elements hydrogen (H) and oxygen (O), and each molecule of water consists of two hydrogen atoms and one oxygen atom. Thus we write the chemical formula for water as "H2O".
Hydrogen itself is also a very important element in the universe. For example, it is the fuel for the Sun, which generates power by fusing (combining) hydrogen atoms into a helium in a process call nuclear fusion. Because it can be obtained from water, as this project demonstrates, the German's call hydrogen "wasserstoff", which literally means "water stuff".
Suppose that you just happen to have some pure hydrogen gas on hand, stored in a container. The hydrogen gas consists of H2 molecules zipping around in a container (hydrogen atoms like to bond together into H2 molecules). If there also happens to be oxygen gas around (O2), and there is always plenty oxygen in the air (air consists of about 20% oxygen), then the oxygen can react violently with the hydrogen gas, such that the hydrogen burns, or combusts, with the oxygen to form water and heat, according to the chemical reaction
2H2 + O2 -> 2 H2O + energy (heat).
Therefore, if you have some hydrogen, you can burn it for fuel to generate heat!
Generating heat, however, is not always the best thing to do, because entropy, which may be thought of as molecular disorder, is created when heat is generated, and that can limit the efficiency of devices that use that heat energy to do useful work (For more info on entropy, see the section on entropy in our Energy Physics Primer). Fortunately, there exists a device called a fuel cell, which can chemically combine hydrogen with oxygen to make electricity. After you complete this project, you may want to also want to cover our project on exploring fuel cells.
Fuel cells can also accomplish what this project demonstrates - electrolysis - which generates hydrogen from water.
Notice from the equation above that, unlike burning a fossil (carbon-based) fuel such as coal, burning hydrogen doesn't produce any byproducts except water, which is environmentally benign. Hydrogen burns clean!
Burning fossil fuels on the other hand, always results in carbon monoxide (CO) and/or carbon dioxide (CO2), which is produced when the carbon atoms combine with oxygen. These compounds are now considered pollutants because they are greenhouse gases - that is, they help trap heat near the Earth's surface, causing the Earth's surface temperature to rise, i.e. global warming.
Burning fossil fuels such as coal also usually releases other pollutants, including sulfur dioxide, mercury, and uranium to the atmosphere, because these substances are usually present to varying extent in fossil fuels. But if we can obtain hydrogen without producing greenhouse gases or these other pollutants, then hydrogen is a better fuel to use than fossil fuels. Many people are now hopeful that a "hydrogen-economy" will soon replace our fossil fuel economy.
There are two obstacles to a hydrogen-economy.
Both of these problems might be surmounted by using synthetic fuels. For example, it is possible, using a catalyst, to combine water, carbon dioxide (extracted from the air), and renewable electricity to make fuels such as methanol, a carbon-based fuel. When this fuel is burned, water and carbon dioxide are produced. But because the carbon dioxide used initially to make the fuel was extracted from the air, the cycle is closed with respect to both water and carbon dioxide, and so won't contribute excess carbon dioxide to the atmosphere. Fuel cells can already use such fuels (either by extracting the hydrogen from the fuel prior to the fuel cell, or even directly in certain types of fuel cells).
Some people are worried that hydrogen might be too dangerous. It is true that hydrogen is a very explosive fuel, but so is natural gas and gasoline. For example, movies commonly depict automobiles burning up after crashing, and explosions involving natural gas are reported in the press from time to time. Two famous disasters involving hydrogen are the explosion of a zeppelin (an airship) called the Hindenburg (in 1937), and the explosion of the Space Shuttle Challenger (in 1986). You may want to study these disasters as a class project. The Hindenburg explosion, although often cited as an example of the danger of hydrogen, is thought by many to have been caused by flammable paint that caught fire from an electrical spark, and so might have caught fire even if the zeppelin had been filled with helium (an inert, nonflammable gas). Moreover, most of the people that died may have done so from coming into contact with burning diesel fuel (which powered the Hindenburg's airplane-type prop-engines) or from jumping of the Zeppelin before it landed.