Researchers are developing new technologies to carry this adaptable fuel in gas, liquid, or solid state.
By Michael Valenti, Senior Editor
The advantages of hydrogen fuel for cars and
trucks keep driving efforts to develop ways of handling it. Hydrogen has the
highest energy content by weight of any fuel—52,000 Btu per pound. As the
simplest and most common element in the universe, though never found
naturally in pure form, hydrogen can be produced from a host of available
sources, including water, natural gas, coal, biomass, municipal solid waste,
or scrap tires.
If burned as a vehicular fuel, hydrogen can be combusted at the high
compression ratios and efficiencies demanded by internal combustion engines.
When combined with oxygen in automotive fuel cells to generate electricity
without combustion, hydrogen fuel raises the car's energy efficiency and
produces only heat and water as byproducts. When hydrogen is burned with air
in an internal combustion engine, some nitrogen oxides are formed, but fewer
than the pollutants generated by fossil fuels, according to the Department
of Energy's Energy Efficiency and Renewable Energy Clearinghouse in
Merrifield, Va.
 Quantum
Technologies' TriShield composite cylinders can hold up to 3 kilograms of
hydrogen at 5,000 psi, which is sufficient fuel for a 200-kilometer journey
in a standard sedan.
Quantum
Technologies' TriShield composite cylinders can hold up to 3 kilograms of
hydrogen at 5,000 psi, which is sufficient fuel for a 200-kilometer journey
in a standard sedan.
Among the obstacles to commercializing hydrogen-powered vehicles—besides
production and infrastructure—is the need for storage systems that can
contain sufficient hydrogen onboard a car to compete with the range and
performance of gasoline-powered autos. Approaches to vehicular hydrogen
storage systems can be as different as gas in pressurized tanks, liquid in
cryogenic vessels, or a solid in metal hydrides.
All three methods are under study: the first at Quantum Technologies Inc.,
the second at Lawrence Livermore National Laboratory, and the third at
ChevronTexaco Ovonic Hydrogen Systems. Each project intends to capitalize on
the strengths and address the limitations of a different hydrogen storage
technique.
Fighting Embrittlement
Vessels pressurized to 5,000 psi can hold sufficient hydrogen for a standard
hydrogen-powered sedan to be driven 200 kilometers. This is the maximum
distance of about 85 percent of the car trips in the United States,
according to a report prepared for the Department of Transportation's Office
of Highway Information Management.
Conventional industrial hydrogen storage tanks are made of common grade
steel and, over time, the gas can migrate into the metal. This makes the
metal brittle, fatiguing it to the point that hydrogen can leak from the
tank. High-quality steel can prevent embrittlement, but will raise the cost
of the tank, and its weight. A premium steel tank holding 3 kilograms of
hydrogen would itself weigh 400 kg, definitely cutting into fuel economy.
Quantum Technologies Inc. of Irvine, Calif., developed its composite
TriShield hydrogen storage cylinder as an alternative that will be
compatible with the cost expectations of the automotive industry, and also
meet safety standards. Quantum specializes in developing advanced fuel
systems, such as the natural gas and propane systems used on General Motors
vehicles, including the J-Car Cavalier Sedan, and the Silverado and Sierra
trucks.
The TriShield contains a cross-linked, ultrahigh molecular weight, modified
polymer liner that has been optimized to withstand temperatures from -40°F
to 190°F. The liner is impermeable. Engineers installed a single boss
opening to minimize leak paths and added a redundant hydrogen seal system to
enhance the cylinder's reliability.
As a further protection against embrittlement, the liner is surrounded by a
carbon fiber inner shell. A hard external shell made of a proprietary
fiber/resin system, and impact-resistant polymer domes on each end, protect
the cylinder from service damage.
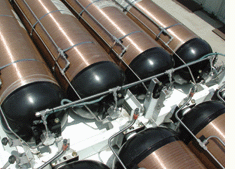 A
series of TriShield tanks are mounted on the roof of an experimental fuel
cell-powered bus in southern California to demonstrate their ability to
store hydrogen fuel safely in field conditions.
A
series of TriShield tanks are mounted on the roof of an experimental fuel
cell-powered bus in southern California to demonstrate their ability to
store hydrogen fuel safely in field conditions.
The TriShield typically measures 22 by 20 inches, but can be 20 feet long
for buses. It holds up to 3 kg of hydrogen pressurized to 5,000 psi, enough
for a 200-km trip in a standard sedan. The cylinder can withstand up to
11,250 psi from accidental overfilling before bursting, and has a fatigue
life of 45,000 cycles, according to ANSI/CSA NGV2-200 hydrogen tank
specifications.
Quantum verified the Tri-Shield's toughness by conducting a series of
rigorous tests under auspices of the European Union's European Integrated
Hydrogen Project. The tests were completed last November. Manufacturers of
storage cylinders must pass the tests to be able to make and sell hydrogen
storage cylinders in Europe.
"These tests included placing the cylinder in a crash car, firing
armor-piercing bullets at it, dropping the cylinder from six feet onto a
concrete surface, placing it in a diesel fire, cycling it thousands of
times, and subjecting the cylinder to extreme cold and to corrosive liquids
encountered in automotive environments, such as battery acids, saltwater,
brake oils, and methanols," explained Neel Sirosh, a mechanical engineer and
director of fuel storage systems at Quantum.
The TriShield passed those tests as well as others for
vibration and shock. "These tests involved using accelerometers on test
vehicles to obtain the vibration profiles of on-the-road service," Sirosh
said. "The vibration profiles were simulated by shake tables in Quantum's
testing labs upon which the tanks were mounted."
TriShield tanks have been installed on a Hyundai Santa Fe sedan being tested
by the California Fuel Cell Partnership, to demonstrate the tank's ability
to feed a fuel cell as well as a combustion engine. Other tanks are mounted
on the roof of a fuel cell bus being developed by Thor/ISC International in
San Diego.
"Quantum also sees opportunities for the TriShield tanks in augmenting
land-based electrical generation," Sirosh said. "Electrolyzers would produce
hydrogen at night, when electrical costs are low, and the gas would be
stored in groups of tanks. That hydrogen would be tapped during the peak
demand in the daytime to generate electricity."
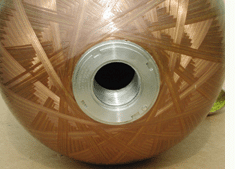 Engineers
installed a single boss opening in the TriShield tank in order to minimize
leak paths for its pressurized hydrogen contents.
Engineers
installed a single boss opening in the TriShield tank in order to minimize
leak paths for its pressurized hydrogen contents.
Quantum recently developed a 10,000-psi version
of TriShield, which is now awaiting certification. According to Sirosh, "We
adapted the material thickness of the tank to accommodate the greater
pressure and increase the tank's energy density by 60 percent. By storing 60
percent more fuel than before in the same-size tank, we make the TriShield
competitive with liquefied hydrogen gas storage."
A proprietary regulator resides in the TriShield to keep only the tank at
high pressure. As a result, the rest of the fuel system, from lines to
engine, can operate under lower pressure, eliminating the precautions needed
for high-pressure gas handling.
This contrasts with gaseous fuel systems that typically deliver fuel at high
pressure to the engine bay, where it is regulated down to the operating
pressure of the fuel-metering device. Thus, the fuel line is filled with
high-pressure hydrogen that can explode if the line is breached.
The TriShield's H2R 5000 regulator is a balanced, single-stage,
piston-sensed device whose inlet accepts hydrogen at pressures ranging from
300 to 10,000 psi, and feeds it to the vehicle fuel system at 50 to 150 psi.
Preventing Evaporation
Cryogenic pressure vessels offer the low weight and volume to accommodate
light-duty vehicles. Five kg of liquid hydrogen would give a high-efficiency
hydrogen fuel cell or internal combustion engine vehicle a 600-km driving
range, according to Salvador Aceves, an ASME member and associate program
leader for transportation at Lawrence Livermore National Laboratory in
Livermore, Calif. Aceves is a past chairman of ASME's Advanced Energy
Systems division.
However, drawbacks of cryogenic storage are the high electrical consumption
required to liquefy the hydrogen, and the evaporative losses that can occur
when the low-pressure tanks are filled and when the car is parked. "It would
take about 65 kilowatt-hours of electricity to liquefy the 5-kg target for
vehicular hydrogen," said Aceves.
The loss of cryogenic hydrogen by evaporation is caused by heat transfer
between the tank and environment. According to computer analyses performed
by Lawrence Livermore, a standard sedan carrying a conventional,
low-pressure (5 bar) cryogenic hydrogen fuel tank would lose 20 percent of
its fuel due to evaporation when a car was driven 50 km per day. Evaporation
losses increased when the car was driven less.
The Lawrence Livermore researchers also modeled the same vehicle, but one
equipped with an insulated cryogenic vessel that can operate at high
pressure-250 bar. "If the car was driven 5 km or more per day, there was
virtually no evaporative loss," said Aceves.
 An
insulated cylinder, designed to contain hydrogen chilled to 20 K, has to
hold up in a bonfire at Authorized Testing Inc. before the tank is deemed
worthy of testing on automobiles.
An
insulated cylinder, designed to contain hydrogen chilled to 20 K, has to
hold up in a bonfire at Authorized Testing Inc. before the tank is deemed
worthy of testing on automobiles.
Based on these findings, Lawrence Livermore is developing insulated,
cryogenic pressure vessels whose fuel flexibility enables them to use
ambient-temperature, compressed hydrogen for short trips, and cryogenic
hydrogen on long trips. "These tanks would require 16 percent less energy
when running on compressed fuel than when using cryogenic hydrogen, and
would experience less evaporative losses when charged with subzero (20 K)
hydrogen by virtue of their insulation," noted Aceves.
Lawrence Livermore determined that commercially available aluminum-lined and
composite-wrapped pressure vessels used for natural gas storage offered the
desired low weight and affordability for automotive fuel storage. They
constructed 1/5-scale tanks for testing.
The researchers coated standard, hydrogen pressure vessels with multiple
layers of metallized Mylar, then built an outer jacket to induce a vacuum
around the pressure vessel. The vacuum jacket reduces air convection and
conduction of heat. Radiant heat is reflected back by the layers of Mylar.
Because a tiny portion of heat can pass through a single layer, Lawrence
Livermore applies about 80 layers of reflective material on its tanks.
 A
.30 caliber armor piercing bullet fired from 50 feet punctured Livermore's
insulated cryogenic hydrogen tank without fragmenting it, thus meeting DOT
and SAE standards.
A
.30 caliber armor piercing bullet fired from 50 feet punctured Livermore's
insulated cryogenic hydrogen tank without fragmenting it, thus meeting DOT
and SAE standards.
Aceves and his colleagues built openings in the outer jackets for
thermocouples, strain gauges, and a capacitive level sensor to measure
pressure, temperature, and level within the tanks. They also equipped the
tanks with safety devices to prevent catastrophic failure in case hydrogen
leaked into the vacuum. These were relief valves that would open if the
pressure limits were exceeded, and rupture discs if the relief valves
failed.
The researchers then subjected the tanks to a variety of Department of
Transportation and Society of Automotive Engineers tests at Lawrence
Livermore's High Pressure Laboratory.
For example, the outer jacket did not leak after being subjected to the
stresses of 1,000 vacuum cycles. A cold shock test involved filling the tank
with compressed helium to 1.2 times the maximum allowable working pressure
for 30 minutes. Then, the vessel was cycled three times in succession from
ambient to low, liquid nitrogen temperatures, and finally, leak tested with
helium pressurized 25 percent above the maximum allowable working pressure.
A mass spectrometer showed no leaks after this phase of testing.
The researchers then tested their insulated pressure vessels by filling them
with gaseous, low-temperature hydrogen and cryogenic, liquid hydrogen. The
tanks did not leak or become damaged.
Whenever the tanks were filled with volatile hydrogen, they were tested at
the national laboratory's High Explosives Facility. "We stayed in a safety
bunker while the tanks were tested in the open," said Aceves.
The laboratory subjected the tanks to a variety of certification tests to
meet the standards of the U.S. Department of Transportation. These included
cycling the tanks, at ambient temperatures, 10,000 times from less than 10
percent of the service pressure to full service pressure.
In a series of environmental cycling tests, the tanks were filled with
gaseous hydrogen to determine their performance in extreme climates. First,
the tanks were cycled 5,000 times from zero to full service pressure, with
an internal tank temperature of 140°F and external ambient air temperatures
in 95 percent humidity. Then, the tanks were cycled 5,000 times from zero to
service pressure at -60°F tank temperature.
The tanks were subjected to 20 thermal cycles, with tank temperatures
ranging from 200°F to -60°F at full service pressure.
All cycling tests were passed successfully, as was the dramatic gunfire test
performed by Authorized Testing Inc. in Riverside, Calif. A .30 caliber
armor-piercing bullet was fired at the tank from a range of 50 feet. Despite
being pierced by the bullet, the tank did not fragment and met the
standards. The tanks were placed in bonfires to demonstrate that their
safety release device would vent gas to prevent an explosion. The tanks also
survived, or suffered acceptable damage, after being dropped from 10 feet
onto hard surfaces.
 Despite
being charred from exposure to a test fire, the Livermore cryogenic tank
retains its structural integrity, a key safety consideration.
Despite
being charred from exposure to a test fire, the Livermore cryogenic tank
retains its structural integrity, a key safety consideration.
In the next few months, Lawrence Livermore will fill its insulated tanks
with liquid nitrogen and subject them to drop and bonfire testing to earn
SAE certification for cryogenic performance. "After we pass the SAE tests,
we want to install the insulated cryogenic tanks on vehicles for field
testing," Aceves said.
Lawrence Livermore already has partners in getting its insulated hydrogen
tanks on the road. They include Structural Composites Industries, a leading
manufacturer of pressure vessels based in Pomona, Calif., and Sunline
Transit of Thousand Palms, Calif. Sunline is the mass transit agency serving
the Palm Springs area and has agreed to install two insulated cryogenic
tanks on pickup trucks. "One, a Ford F 250, will carry liquefied natural
gas, and the other, a Ford Ranger, will hold liquid hydrogen," Aceves said.
The project is funded by the Department of Energy's hydrogen program and the
South Coast Air Quality Management District.
Hydrides on a Diet
Compressed gas tanks store hydrogen as a gas, and their cryogenic
counterparts store it as a liquid. A less familiar method of storing
hydrogen is as a solid in metal hydrides, alloys of rare earth, transition
metal, and magnesium. These granulated materials absorb hydrogen. Because
the hydrogen is chemically bonded to the alloys, heat is required to release
it.
Among the advantages metal hydride has in fuel storage is its compactness.
It holds the target fuel value of 5 kg of hydrogen in one-third the volume
of a gaseous hydrogen tank at 5,000 psi, and one-quarter the volume of a
hydrogen tank at 3,600 psi.
Metal hydrides are also inherently safe. Hydrogen is held in the hydrides at
a low pressure-less than 200 psi-at ambient pressure. In case of a crash,
the hydrides will not discharge their hydrogen cargo because it is
chemically bonded to the hydrides in a solid state.
These pluses are literally outweighed by metal hydrides' biggest drawback:
They are heavy. "A metal hydride storage system that can hold 5 kg of
hydrogen, including the alloy, container, and heat exchangers, would weigh
approximately 300 kg, which would lower the fuel efficiency of the vehicle,"
according to Rosa Young, a physicist and vice president of advanced
materials development at Energy Conversion Devices in Troy, Mich. "The
challenge is to formulate a high-capacity metal hydride alloy whose kinetics
and thermodynamic properties, along with more efficient heat exchangers, can
be used to make a lightweight hydride storage system," Young explained.
ECD specializes in developing the materials and components for alternative
and advanced energy systems for homes, vehicles, and industry. The company
formed a 50-50 joint venture with Texaco Energy Systems Inc. at the end of
October 2000 to develop and commercialize lightweight metal hydride storage
systems for fuel cell vehicles and for internal combustion cars that burn
hydrogen. The venture, which was originally called Texaco Ovonic Hydrogen
Systems LLC, is now known as ChevronTexaco Ovonic Hydrogen Systems.
 Researchers
at ChevronTexaco Ovonic Hydrogen Systems are studying the charging speed and
desorption rates of the metal hydrides they have developed to store
vehicular hydrogen in a solid state.
Researchers
at ChevronTexaco Ovonic Hydrogen Systems are studying the charging speed and
desorption rates of the metal hydrides they have developed to store
vehicular hydrogen in a solid state.
Young and her colleagues are testing a 2.5-kg hydrogen storage tank using
a proprietary metal hydride at the company's laboratory. A full-size,
onboard system will consist of one or two tanks. A smaller, European economy
car would use one tank to feed a fuel cell that would generate about 35 kW
of electricity, while an American fuel cell economy sedan would use two
tanks to generate the requisite 70 kW. The fuel supply will provide a
driv-ing range of more than 200 miles.
Researchers feed hydrogen directly into the tanks, where it is absorbed by
the powdered alloy. As the hydrogen gas is absorbed during charging, the
metal hydrides generate heat that is removed by water. When a fuel cell car
is driven, the waste heat from the cell is used to release the hydrogen.
Because fuel cells generate a large amount of low-grade heat when they
operate, Ovonic designed a metal hydride tank to capture a portion of the
waste heat of the fuel cell as well. "Our system is part of the car's
radiator, so that the radiator's weight and size can be reduced in size,"
Young said.
The Ovonic researchers are studying two key parameters of their storage
system, namely, charging speed and the desorption rates of hydrogen during
simulated driving conditions. "We are modifying the kinetics of the material
by metallurgy and by designing a high-efficiency heat exchanger so that it
can absorb 5 kg of hydrogen in 10 minutes, and desorb it at ambient
temperature," noted Young. "We are also calibrating the use of the waste
heat to provide the required release rate of hydrogen for driving."
Young said that her company will have a full-scale prototype on the road for
vehicle demonstration within the present year. Because of ChevronTexaco's
international presence, the joint venture is also working on hydrogen
service stations using metal hydrides for hydrogen bulk storage. It is
possible that the prototype onboard system and bulk storage system will be
tested in the United States, Europe, and Asia.