How does
the earth radiate?About one
half of the solar energy that reaches the atmosphere's outer limits
from space actually hits the surface of the earth. The other half of
solar insolation is already reverberated (reflected) or taken up
(absorbed) earlier on its way through the atmosphere. It it thus by
the remaining half that reaches the ground that the surface of the
earth is heated. Every heated body, though, radiates by itself,
proportional to its temperature. We are familiar with this experience
in everyday life regarding, e.g., a stove plate. The more energy (in
the shape of electric power) we put in, the hotter it becomes and the
more clearly we feel the heat radiated when we hold one hand above the
plate.
According to the laws of physics, one is able to
calculate the range of wavelengths in which radiation is emitted at a
certain temperature of the stove plate or, more generally, a heated
body. The many-colored area in the ensuing illustration shows us how
the radiation of heat is distributed if the temperature of a body is
of the order of 280 Kelvin (+7° C). This almost corresponds to the
earth's mean global temperature at its surface. The illustration
shows a spectrum of the so called infrared radiation approximately
between 400 and 1800 cm-1 (the unit "cm-1"
simply represents a way to describe the energy of infrared radiation
by means of so called "wave numbers" referring to the number of wave
peaks that can be fitted into an interval of the width of one
centimeter).
red+yellow+blue =
total radiation of the earth at +7° C in the range between 400 and
1800 cm-1.
blue = radiation that
is absorbed by greenhouse gases.
yellow = radiation that is
allowed to pass by greenhouse gases.
(red = absence of an
absorption spectrum due to technical reasons concerning the
measurements.)
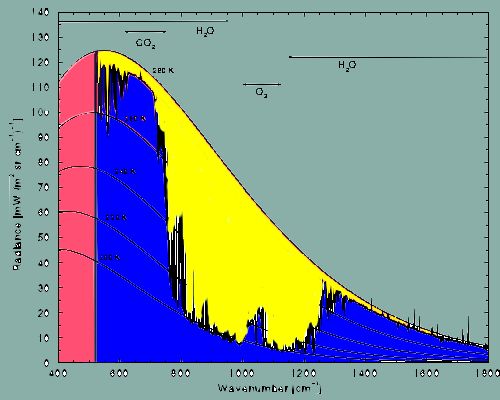
But: in the range between approximately 500 and
1800 cm-1, depicted in blue, the illustration shows the
total amount of absorption caused by the most important atmospheric
trace gases, namely water vapor (H2O), carbon dioxide (CO2),
and ozone (O3).
These so-called 'trace gases' - this term refers
to their relatively insignificant abundance as compared to the
influence these compounds exert to the atmosphere - constitute, so to
say, the wool of the sweater in which the earth is wrapped up. Here,
the emitted heat catches on immediately. Only at distinct energies
(depicted in yellow color) the radiation is able to escape through the
atmosphere into space without being moderated.
It can clearly be obtained from the illustration
that water vapor absorbs over a wide range of the spectrum.
Water
vapor is the most important greenhouse gas!
In a very rough approximation the following
trace gases contribute to the greenhouse effect:
60% water vapor
20% carbon dioxide (CO2)
The rest (~20%) is caused by ozone (O3),
nitrous oxide (N2O), methane (CH4), and several
other species.
|
State of the
Art
The contribution of water vapor to the
anthropogenic greenhouse effect (i.e., that portion of greenhouse
warming caused exclusively by humans) is still controversial. At
numerous environmental conferences, greenhouse gases, such as CO2
and methane (CH4), are discussed primarily while many
times the role of water vapor in both its natural and
anthropogenic aspects remains unmentioned. Yet water vapor not
only holds the pole position concerning the natural greenhouse
effect, but also participates in the additional absorption of heat
in the atmosphere which is exclusively caused by human activities.
We're not speculating that we would blow
enormous amounts of water vapor into the air and enhance the
greenhouse effect. On the contrary, the concerns are for
so-called "secondary effects". That is: if the average
temperature of atmospheric layers near to the ground, as a
consequence of anthropogenic CO2 and methane emissions,
is rising, then the evaporation of water is increased. Henceforth
more water vapor will get into the air, and this additional
abundance of water vapor will also absorb more heat.
It remains uncertain, though, which
concentrations at which locations and at which altitudes in the
troposphere will contribute the most to greenhouse warming. In
addition, it is unclear how this surplus of water vapor will alter
the warming process of the earth.
|
One of the major problems in climate
research at present is the fact that we still cannot
realistically reproduce the formation of clouds in
currently available climate models. Therefore an exact
prediction of the influence exerted by water vapor and a
prediction of the warming on the whole, remains very
doubtful. This area of research calls for an enormous
amount of scientific work.
|
We conclude:
Due to the
so called "greenhouse effect" - caused by atmospheric
trace gases such as carbon dioxide (CO2),
ozone (O3), and water vapor (H2O)
- infrared radiation from the earth is stored
temporarily in the atmosphere. Of all these trace
gases, water vapor represents the most important
constituent. It contributes to the natural greenhouse
warming process by approximately 60%. Water vapor
amplifies the anthropogenic contribution to greenhouse
warming through a positive feedback. This
amplification is counteracted by the increased
reflection off clouds. How these two factors combine
in the real atmosphere still remains an open question. |
|
|
|
