On Fire
by Rick Groleau
Whether it's in the form of an exploding firework or a burning cigarette,
combustion is merely some material rapidly combining with oxygen. Well, maybe
that's stating it too simply. Combustion turns out to be a complex interaction
between molecules -- even the burning of a simple five-atom molecule can involve
more than 100 individual chemical reactions. And if you take a look at the
burning of organic matter such as tobacco and wood, which contain long molecules
of intricately arranged atoms, the interactions are substantially more involved.
This feature lets you explore the basics of combustion, including how a fire
ignites, how a molecule's atoms rearrange themselves during combustion, and what
a flame is made of.
On
Fire
Step Through the Reaction
Back
to Chain Reaction

Step 1
The chemical reactions of hydrogen combustion occur because certain atoms and
molecules are very reactive -- they are unstable on their own and quickly
combine with other molecules or atoms.
Adding the single hydrogen atom to the mixture of oxygen and hydrogen gas, as
you did, led to a series of chemical reactions.
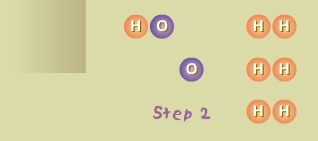
Step 2
The single hydrogen atom (H) reacted with a molecule of oxygen (O2),
producing a molecule of hydroxyl radical (OH) and an atom of oxygen (O). Both OH
and O are also highly reactive.
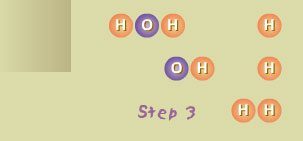
Step 3
The OH molecule reacted (very rapidly) with a hydrogen molecule (H2),
producing a water molecule (H2O) and another hydrogen atom (H). The
oxygen atom liberated in Step 2 reacted with H2, producing new OH and
H.

Step 4
The new OH molecule reacted with another H2 molecule, producing
another molecule of water (H2O) and yet another hydrogen atom (H).

Steps 5 -
The three hydrogen atoms liberated by this reaction quickly reacted with three
other O2 molecules, which resulted in the liberation of nine other
hydrogen atoms. Those nine liberated 27 more, then those 27 liberated 81, and so
on. This is known as a branching chain reaction.
Shuffle Atoms

Methane (CH4) is a clean-burning fuel that is the main constituent of
natural gas, the type of gas piped into homes. The combustion of methane (CH4)
is similar to that of hydrogen, though with methane, many more chemical
reactions take place before stable molecules form.
When ignited, a molecule of methane reacts with two molecules of oxygen (O2).
The end result is three new molecules: two molecules of water (H2O)
and one of carbon dioxide (CO2).





