|
From black currant to green current
Introduction
Every day we can see the most fascinating “solar cells”, for example green spinach, algae, and green leaves on trees. All of these convert sunlight by means of photosynthesis into energy containing nutrients like sugar, which are important for life. Why couldn’t we use spinach with a little bit of sunlight to generate electricity? Is it really possible to generate “green current" out of blackcurrant?
The answer to these questions is given by "the nanocrystalline dye solar cell" which is also called "organic solar cell", or after the inventor, "Grätzel cell". This new type of solar cell partly bypasses the photosynthesis and makes a shortcut conversion into electrical current. This is what is called photovoltaic power generation. The inner part of this solar cell consists of molecules similar to chlorophyl, the same dye which is present in green leaves. When it comes to direct conversion of sunlight into electricity, however, it is better to use other dyes. Professor Grätzel has developed a synthetic dye which is able to convert a large part of the sunlight into electrical current. Apart from the synthetic dyes natural dyes such as chlorophyl, can also do the job. The best natural dyes presently known are the anthocyanates. Anthocyanates are the purple-red dyes found in for example black berries, raspberries, hibiscus leaves and blackcurrant.
At present these solar cells based on synthetic dyes are made and studied in the laboratory. The functioning of this solar cell is explained in the section "Working principle".
The aim of Man Solar is to show this ingenius dye solar cell in its most natural form. Man Solar has modified the components of the Grätzel cell so that a solar cell can be built with the aid of hibiscus leaves, black berry juice or black currant, "toothpaste titanium dioxide", a pencil, a little bit of iodine and electrically conductive glass. Fresh black berries, cherries or raspberries are preferable. Fruit juice from the super market based on these kinds of fruit can also work. To enhance shelf-life we included dried hibiscus leaves for the experiments. |
Photosynthesis: fundamental metabolic reaction of organisms containing chlorophyl (plants, algae) in which glucose and oxygen (O2) are formed out of carbon dioxide (CO2) and water (H2O) under conversion of light energy into chemical energy.
Photovoltaic power generation: direct conversion of (sun)light into electrical current. The old Greek word 'photo' means light and voltaic is derived from the name of the Italian physician Alessandro Volta. The unit 'Volt' for electrical potential is named after him.
Working Principle of the dye solar cell
Before the working principle of the dye solar cell is explained some important definitions are given.
|
TCO |
Transparent conducting oxide, a thin coating which is electrically conducting like a metal wire. The TCO-coating is present on one side of the glass only. Most of the light of the sun can pass through the glass and the TCO-layer without loss. |
|
Photon |
Light particle, of which the energy depends on the wavelength (colour) of the light. |
|
Electron |
Electron: elementary particle which is part of an atom, it is negatively charged. Electrical current consists of electrons moving through a conductor, like a metal wire, from the negative to the positive pole of a current source (i.e. a battery or a solar cell). |
|
Titanium dioxide |
Mineral that is commonly used as a white pigment in paint, tooth paste, fill material in pills and many other applications. |
|
Electrolyte |
Electrically conducting liquid, in this case ions rather than electrons are responsible for the charge transport through the liquid. |
|
Ion |
Atom which is missing one or more electrons or has one or more electrons too many and consequently is positively or negatively charged. Originates from the Greek word Ion, 'Wandering'. |
|
Graphite |
Crystalline carbon that can conduct electricity and functions as a catalyst. |
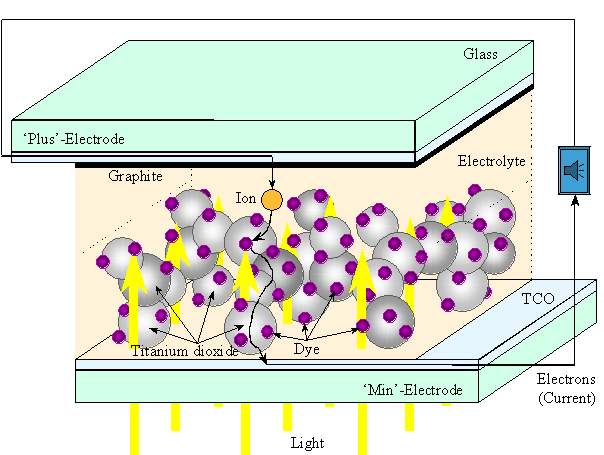
The dye solar cell consists of a thin layer (appr. 10 micrometer thickness) of randomly stacked titanium dioxide particles (appr. 20 nm in diameter) to which organic dye molecules are chemically attached. The titanium dioxide particles form a three dimensional network which is electrically conducting when illuminated. Stacked titanium dioxide particles are attached to a TCO coated glass substrate. The TCO layer consists mainly of coated tin oxide on glass and is essential for transporting the current produced by the solar cell to the power consuming device, for example a calculator. The TCO substrate with titanium dioxide and attached dye molecules is called the photo electrode being the negative pole of the solar cell. To complete the solar cell (and the electrical circuit) also a counter electrode and an electrolyte liquid are required. This will be explained later.
The transformation of the energy of light into electrical energy works as follows in the dye solar cell. A dye molecule absorbs a small amount of light. The energy present in this light is transferred to one electron in the dye molecule. Upon transfering this energy the electron becomes mobile and is able to leave its defined bond. The electron possesses enough energy to migrate through the titanium dioxide and the TCO to the electricity consuming device. A closed circuit is required in order to have an electrical current run. This means that electrons, after having transferred their energy to a consuming device, must return to the spot where they were released. To realize this, a counter electrode (positive pole) is required which absorbs the electrons from the electricity consuming device. These electrons return via the electrolyte to the dye molecules that are missing an electron. The counter electrode absorbs the electrons and transfers them to ions present in the electrolyte liquid. To let this process run efficiently, a catalyst is required. The catalyst used is a layer of graphite for example from a pencil, deposited onto the TCO of the counter electrode. The "charged" ions carry the electrons through the liquid and the pores of the titanium dioxide network until they meet with a dye molecule missing an electron. The electron is then transferred from the ion to the dye. This final step closes the electrical circuit and the dye is ready again to carry out the process of transforming light into electricity.
The dye solar cell was invented by the group of Prof. Michael Grätzel in Switzerland and is often referred to as Grätzel-cel. After the discovery in 1991 [1] many research laboratories have investigated natural alternatives to the synthetic dye [2]. Since their initial discovery these cells have been recognized as valuable educational tools [3]. Since January 1995 ECN [4] has concentrated investigations upon the technological aspects of the dye solar cells.

A dye solar cell manufactured by the Energy research Centre of the Netherlands (ECN).
References
- B. O'Regan, M. Grätzel, Nature 353, 737-739 (1991).
- A. Kay, M. Grätzel, J. Phys. Chem. 97, 6272 (1993).
- G.P. Smestad, M. Grätzel, J. Chem. Educ. 75, 752 (1998).
- www.ecn.nl