Making the gascheaply will get around the first roadblock to widespread hydrogenpoweredcars.
 |
|
The Titan H2 generator from Teledyne Energy Systems Inc., Hunt Valley, Md., electrolyzes an alkaline solution into H2 and O2 and can make hydrogen at 5,720 scf/h with 99.7% purity. Input power is 460 Vac, inverted to 1.8 to 2.0 Vdc per cell at high current. |
 |
|
A 5x5-ft solar concentrator at Shec Labs generates hydrogen in a two-stage catalytic process. CEO Beck says this setup can generate the gas at 5 liter/min. The company says their process runs at a relatively low 750C. |
 |
|
The 18-in.-diameter solar concentrator can create temperatures in excess of 750C, enough for a low-rate H2 generator. |
 |
 |
|
Commercial electrolysis works in a pressurized cell generating H2 and O2 at over 300 psi. Stuart Energy, Ont., Canada, uses a Vandenborre IMET Technology — a pressurized alkaline electrolyzer — that generates highpurity hydrogen at pressures to 363 psi. |
 |
|
The Hogen 40 hydrogen generator from Proton Energy, Wallingford, Conn., manufactures 99.9% pure hydrogen at 200 psi. |
Senior Editor
Hydrogen holds fantastic promise as a plentiful, clean-burning fuel and an eventual replacement for gasoline. Environmentalists like it because it might trim the amount of greenhouse gas spewed by the nation's automobiles. (The combustion of hydrogen produces only water.) Engineers like it because it's new technology that will need fueling with lots of ideas and design work. One futuristic idea places wind turbines in the windiest part of the country electrolyzing water and pumping it into a national grid. And cynics like the idea of a hydrogen economy because it lets them snicker while pointing out the hurtles that must be cleared to get there. Even now there are several commercially active methods for producing the gas, although most of the 9 million tons produced annually are for on-site industrial use.
The Federal government and energy companies have big plans for hydrogen. General Motors, Honda, and others have prototype fuel-cell cars running around a few cities, mostly out West. California has about 10 H2 refueling stations. And President Bush has proposed $1.2 billion for research.
There are just a couple speed bumps in the road to a hydrogen economy. First, current production techniques are outrageously expensive. For example, on the low end, the energy equivalent to a gallon of gas costs over $7. Add costs for storage and transportation, and the price skyrockets. What's more, hydrogen molecules are so small they leak out the tiniest cracks, and the gas weakens steel, a process called hydrogen embrittlement. And there are suspicions that H2 loose in the atmosphere might eat more of the ozone layer. So new materials will be needed for safe handling. Still, the engineering challenges of making H2 competitive with gasoline will make for an interesting couple decades.
Transitioning to a hydrogen economy will be at least a 20-year evolution, if at all. The most promising production methods so far include electrolysis, high-temperature electrolysis, chemical reformations, and biological processes.
WHAT LOOKS PROMISING
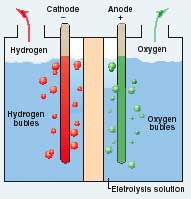
It would be difficult to imagine a hydrogen-generating process
simpler than electrolysis. Drive dc current through electrodes
submerged in an electrolyte, such as saltwater, and H2 bubbles up on one
electrode and oxygen on the other. Several commercial hydrogen
generators use a variation of that process. Most use a high-pressure
chamber to boost efficiency and a membrane between the electrodes to
keep gases from mixing. Although intended for industrial applications,
companies such as Proton in Wallingford, Conn., have developed a
washing-machine-sized H2 generator capable of 40 scf/hr at 200 psi. But
at $65,000/unit, they are unlikely to find their way to garages soon.
Companies such as Teledyne Energy Systems Inc., Hunt Valley, Md., aim to boost the output pressure of today's electrolysis unit. "Right now, our equipment produces hydrogen at up to 230 psi. We want to boost output pressure to 2,000 psi and higher to eliminate secondary compression operations," says Chris Kuehn, director of commercial business with Teledyne. Kuehn adds that part of the Department of Energy's cooperative agreement with his company is to suggest how it might turn out 10,000 hydrogen-generating stations/year, just in case the H2 economy takes off.
Carolyn Elam, senior project leader for hydrogen production at the National Renewable Energy Laboratory (NREL), Golden, Colo., suggests that generating the H2 needed by citysized fleets will call for low-cost electricity. Conventional electrolysis is about 75% efficient for hydrogen (6.4 kW-hr/m 3 according to Teledyne) and Elam expects this to climb over 80%. An NREL report notes that before conventional electrolysis can be considered a viable energy source, the cost and efficiency of commercial electrolyzer systems must improve.
There are two basic types of electrolyzers available today. One uses an aqueous solution of alkaline electrolytes like potassium hydroxide. This technology is the basis for large commercial electrolyzers. The other uses a solid ion-conducting electrolyte membrane. Both require catalysts that include precious metals such as platinum. One goal is to reduce the catalyst loading and improve the durability of the system.
Elam suggests that electrolysis powered by wind turbines could be the most viable in the near term. The setup makes good use of variable winds because hydrogen can be stored and used when needed.
Thermal methods using solar concentrators provide another
route to H2 production. For example, Shec Labs, Saskatoon, Sask.,
Canada, has a system that uses a solar concentrator to generate steam
and hydrogen at a relatively low temperature in a proprietary multistage
catalytic process. "Right now it uses catalysts at two stages to produce
intermediate gases, but H2 in the end," says Thomas Beck, CEO of the
lab. " Hydrogen purity is about 90% and a filtration stage can improve
on that figure." Small-scale tests from a 5 X 5 ft
concentrator has it making about 5 liters of hydrogen/min. Beck believes
the system can scale to any size.
The Shec process requires temperatures of only 750C, one easily reached by concentrating sunlight. "The process has operated at temperatures as low as 400C," says Beck. "In comparison, direct thermal-water splitting normally requires temperatures of 2,000C to begin the reaction and 5,000C to optimize it." Beck adds that the Shec process removes major cost components usually needed to thermochemically extract H2 from water.
In addition, the lab has developed advanced solar concentrators capable of focusing sunlight to 5,000 times its normal intensity. This allows for the efficient and economical collection of solar energy which can then be used for heating, distillation, air conditioning, power generation, and hydrogen production.
"We are also generating electricity using concentrator technology," he says. "It's photovoltaic technology, but we reduced the amount of semiconductor material required by a factor of 500. The result produces electricity at a low cost, and that can be used for traditional electrolysis."
As system temperatures decrease, the energy lost as radiant heat also drops. This yields higher energy-conversion efficiencies. The added benefit of reduced temperatures is in the use of lower cost materials which are more readily available than the expensive materials required for high temperatures.
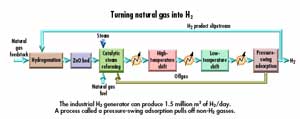
"Another H2 production process uses steam to reform natural gas and accounts for about 95% of the gas' production," says Elam. In a nutshell, a small amount of hydrogen pretreats the natural gas, turning sulfur compounds to H2S, which is removed in a ZnO bed. After pretreatment, natural gas and 380-psi steam go to a steam reformer. The mixture goes into high and low-temperature shift reactors where a water-gas shift reaction converts 92% of the CO into other gases and H2, and removes them from the product stream. Gas reforming also produces 700-psi steam that can be used elsewhere. By one estimate, this process is about 89% efficient.
Biomass may also contribute to hydrogen stocks. Biomass can be thermally processed to generate a hydrogen-rich gas from which pure hydrogen is extracted. Also, many microbes generate hydrogen as waste products. Anaerobic digestion can also be used to make methane that can be reformed into H2. "Industries such as agriculture, pulp and paper, and lumber have a lot of the needed clean waste streams, says Elam.
Electrolysis can also be powered with photovoltaics. However the cost of photovoltaicgenerated electricity remains high. One option to lower the cost of solar-based water splitting is to combine the electricity generation and water splitting into a single device.
Multijunction cell technology developed by the PV industry can be used for photoelectrochemical (PEC) light-harvesting systems that generate sufficient voltage to split water and are stable in a water/electrolyte environment. Theoretical efficiency for tandem junction systems is 42%, practical systems could achieve 18 to 24% efficiency, and lowcost multijunction amorphous silicon (a-Si) systems could achieve 7 to 12% efficiency. The advantage of a directconversion hydrogen-generation system is that it eliminates most of the costs of the electrolyzer, and also has the possibility of increasing the overall efficiency of the process.
Similarly, biological systems can capture the power of the sun to produce hydrogen from water. Certain photosynthetic microbes produce hydrogen in their metabolic activities. By employing catalysts and engineered systems, hydrogen production efficiency could reach 24%. Although photobiological technology holds great promise, a major hurdle will be to overcome the issue of oxygen sensitivity. Oxygen is also produced during water splitting, and this oxygen shuts off the activity of the hydrogen-evolving enzyme. Researchers are addressing this issue by looking for naturally occurring organisms that are more tolerant of oxygen, and by creating new genetic forms of the organisms that can sustain hydrogen production in the presence of oxygen.
| HOW A FEW ENERGY SOURCES STACK UP | |||||
| Energy | |||||
| source | |||||
| CONVENTIONAL | |||||
| Natural gas | |||||
| Cool | |||||
| Nuclear | |||||
|
RENEWABLE
|
|||||
| Wind | |||||
| Concentrating solar power | |||||
| Biomass | |||||
|
Researchers at NREL who generated the table say natural-gas supplies are limited and at best can be only a near-term part of the H2 story. Natural gas is also a poor choice for fueling hydrogen or electric vehicles (EVs) because of increasing emissions, costs, and lower efficiencies than other methods. Producing hydrogen from coal is hobbled by concerns about global climate change and health risks. Nuclear will need a big turnaround in public opinion. Wind is the cheapest renewable source with great potential for hydrogen production at the turbine. Compared to today's costs of hydrogen produced from natural gas, adequate cross-country electric or pipeline transmission is a major constraint. |
|||||
Hijacking the hydrogen revolution
Hydrogen looks like a good bet to replace gasoline as a predominant
automotive fuel. The only question is when. Forecasts get blurry looking
even three years out, so it's hard to say how cars will be fueled in
2020. Hybrid autos work so well, most small cars and trucks might be
some variation of gasoline/battery power by the end of the decade. It's
also reasonable to assume battery technology will improve, possibly to
the point of providing a 150-mile range or better. If that happens, cars
getting 50 mpg on gasoline will look downright wasteful. Generating,
storing, and using electricity could become so efficient with a high
energy-density battery that the hydrogen economy will be pushed out
several more decades.
HYDROGEN BY THE NUMBERS
H2 is colorless, odorless, tasteless, and nontoxic at atmospheric
temperatures and pressures. But the figures are solid.
Boiling point: -423°F (-253°C)
Density: 0.0899 kg/m 3
Energy content: 52,000 Btu/lb, the highest per unit of weight of any known fuel. By comparison, gasoline has about 18,000 to 20,000 Btu/lb.
Electrolysis efficiency: 4.8 kW-hr/m 3 of H2 (Stuart Energy)
Cost: $18 for 250 ft 3 of H2 at 2,265 psi.
Efficiency of gas reformation processes: about 89%
Range from 100 scf of H2: 6 to 12 miles from an internal combustion (IC) engine, 12 to 24 miles from a fuel cell.
H2 cost/mile for an IC engine: $0.60 to $1.20
Gasoline cost/mile ($2/gallon, 25 mpg): $0.08
A new take on the Hindenburg
Critics of wider hydrogen use often point to the explosion of
the German zeppelin Hindenburg as proof the gas is untrustworthy
and dangerous. But according to the Department of Energy and their Quick
Facts brochure, the air ship did not blow up because hydrogen was used
as a lifting gas. The real culprit was the paint coating the ship's
skin: It contained a highly flammable cellulose nitrate or acetate for
rigidity. In addition, the skin was coated with aluminum to reflect
sunlight and keep the H2 from heating. An electrical discharge ignited
the skin and eventually the hydrogen. The H2 burned quickly, floating
upward and away from the people, most of who died by jumping or falling
to the ground. Only two of the 37 who perished died from burns and those
were credited to the burning coating and onboard diesel fuel.