Researchers Develop Method for Enzymatic Production of Hydrogen from Biomass at High Yields
23 May 2007
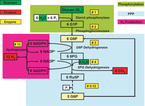
|
| The synthetic metabolic pathway for conversion of polysaccharides and water to hydrogen and carbon dioxide. Click to enlarge. |
Researchers at Virginia Tech, Oak Ridge National Laboratory (ORNL), and the University of Georgia have developed a novel method using multiple enzymes as a catalyst for the direct, low-cost production of hydrogen from biomass.
Applying the principles of synthetic biology, the researchers use a combination of 13 enzymes to form an unnatural enzymatic pathway to completely convert polysaccharides—e.g., starch and cellulose—and water into hydrogen at a yield higher than the theoretical yield of biological hydrogen fermentations. Their work is described in the 23 May issue of PLoS ONE, the online, open-access journal from the Public Library of Science.
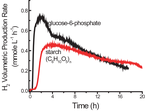 |
| Hydrogen production from either 2 mM G-6-P or 2 mM starch (glucose equivalent) using the new method. Click to enlarge. |
Starch is a high energy-density carrier, with 14.8 H2-based mass%. (The DOE long-term target for hydrogen storage is 12 mass%.) The enzymes, when added to the biomass solution, use the energy in the polysaccharides to break the water up into carbon dioxide and hydrogen.
A membrane bleeds off the carbon dioxide and the hydrogen is used by a fuel cell to create electricity. The water byproduct is recycled for the starch-water reactor. Laboratory tests confirm that it all takes place at low temperature—30° C—and atmospheric pressure. The researchers estimated the cost of hydrogen production using their method of approximately $2/kg.
The stoichiometric reaction is:
C6H10O5 (l) + 7 H2O (l) → 12 H2 (g)+6 CO2 (g)
The overall process is spontaneous and unidirectional because of a negative Gibbs free energy and separation of the gaseous products with the aqueous reactants.
The vision is for the ingredients to be mixed in the fuel tank of a car. A car with an approximately 12-gallon tank could hold 27 kg of starch, which is the equivalent of 4 kg of hydrogen. One kg of starch will produce the same energy output as 1.12 kg (0.38 gallons) of gasoline.
The research was based on earlier work by Y.H. Percival Zhang, assistant professor of biological systems engineering at Virginia Tech pertaining to cellulosic ethanol production (earlier post) and the ORNL and University of Georgia researchers' work with enzymatic hydrogen production.
One of the team, Michael W.W. Adams of the University of Georgia UGA, is co-author of the first enzymatic hydrogen paper in Nature Biotechnology in 1996. The researchers were certain they could combine the processes.
In nature, most hydrogen is produced from anaerobic fermentation. But hydrogen, along with acetic acid, is a co-product and the hydrogen yield is pretty low—only four molecules per molecule of glucose. In our process, hydrogen is the main product and hydrogen yields are three-times higher, and the likely production costs are low—about $1 per pound of hydrogen.
What is more important, the energy conversion efficiency from the sugar-hydrogen-fuel cell system is extremely high—greater than three times higher than a sugar-ethanol-internal combustion engine. It means that if about 30 percent of transportation fuel can be replaced by ethanol from biomass as the DOE proposed, the same amount of biomass will be sufficient to provide 100 percent of vehicle transportation fuel through this technology.
—Y.H. Percival Zhang
The next step for the team is to increase reaction rates and reduce enzyme costs.
Resources:
- “High-Yield Hydrogen Production from Starch and Water by a Synthetic Enzymatic Pathway”; Y.H. Percival Zhang, Barbara R. Evans, Jonathan R. Mielenz, Robert C. Hopkins, Michael W.W. Adams; PLoS ONE 2(5): e456. doi:10.1371/journal.pone.0000456
Comments:
Truly amazing! If this is true it will be very big. And I like the idea of filling the tank with cellulose flour shaken with water. Completely non-toxic and non-flammable. I would like to know more about the enzymes. Would you need a separate tank for that and how much would be needed a gallon?
Green Car Congress © 2006 BioAge Group, LLC. All Rights Reserved. To subscribe or visit go to: http://www.greencarcongress.com