-
August 27, 2007 - Volume 85, Number 35
- pp. 16-22
|
Tapping The Sun
Basic chemistry drives development of new low-cost solar cells
Mitch Jacoby
"MORE ENERGY—in the form of sunlight—strikes Earth in one hour than all of the energy consumed by humans in an entire year."
It's a staggering statistic and just one of the facts that Nathan S. Lewis lists when he makes the case for the importance of solar energy research. Ready with data and calculations to bolster his argument, Lewis, a chemistry professor at California Institute of Technology, runs down the list of alternatives to fossil fuels—nuclear, wind, hydroelectric, biomass, and geothermal—and argues that the sun is the only viable and renewable source capable of satisfying humanity's great thirst for energy. Currently, on a global scale, energy usage is on the order of 13 terawatts (13 trillion W or 13 trillion joules per second), of which roughly 85% is generated by burning fossil fuels.
 Mitch Jacoby/C&EN
Mitch Jacoby/C&EN
Handy Device Yang (right) and
graduate students Greene (left) and Benjamin Yuhas (center)
inspect a nanowire solar cell.
|
Showering Earth with an energy flow of some 120,000 TW, the sun
would appear to be a limitless non-carbon-emitting energy fountain
capable of meeting worldwide energy demands. "There's no doubt that we
have ample energy resource in the sun," Lewis stresses. The challenge,
he says, is figuring out how to tap into it inexpensively. That challenge has been driving researchers to develop new materials and strategies for designing photovoltaic systems that convert sunlight into electricity. In addition to exploring new methods for reducing the cost of solar cells based on silicon, the traditional photovoltaic material, scientists have been experimenting with other semiconductors, inorganic nanocrystals, organic polymers, and a host of other light-sensitive materials. |
For decades, high-purity crystalline silicon solar cells have provided electrical power for a number of high-end applications such as space vehicles. Although such cells' performance and longevity testify to the technology's reliability, the high costs of the crystal-growth and manufacturing processes used for fabricating top-of-the-line photovoltaic modules (multicell units) make those types of devices too expensive for widescale use. To reduce costs, some manufacturers have turned to less expensive forms of silicon including polycrystalline, nanocrystalline, and amorphous silicon. In addition, they've developed cost-saving strategies that reduce the quantity of silicon used per cell.
California-based SunPower, for example, has designed solar cells in which the thickness of the polycrystalline silicon wafer (the active photovoltaic layer) measures roughly half the 300 µm or more that is typical of other manufacturers' cells. The overall cell design relies on special reflectors and other light-trapping features to make efficient use of the thin silicon wafers.
Another approach to minimizing silicon usage is based on a micromachining method designed to increase the fraction of a wafer that can be used to fabricate solar cells. Rather than starting with the thinnest wafers possible, this fabrication process starts with relatively thick disk-shaped wafers of single-crystal silicon and calls for slicing paper-thin rectangular sections from a wafer in a direction perpendicular to the wafer's face. The procedure greatly increases the silicon surface area and leads to a large number of long and very thin (about 50 µm) silicon slices or slivers, each of which is then fashioned into a solar cell. Researchers at Australian National University, in Canberra, developed this sliver design, which is being commercialized by Origin Energy, based in Adelaide, Australia.
|
EVEN AS INVESTIGATORS devise new procedures to reduce the cost of silicon solar cells, the high price of photovoltaic-derived electricity, relative to electricity produced by burning fossil fuels (just a few cents per kilowatt-hour), stands as a major hurdle to wide-scale implementation of solar technology. Estimates of the price difference vary. A factor of 5 to 10 is often cited and depends on several assumptions, including the cost of storing and distributing the power and the lifetime of the power-generating device.
|
 Australian National University
Australian National University
thin is in Developed at Australian National University, 50-µm-thin sliver solar cells are designed to conserve costly crystalline silicon. |
The upshot, as Lewis points out, is that currently, photovoltaic sources contribute only about 0.001% of the total energy supply. Lewis discusses many of the issues associated with energy cost, storage, and consumption in a pair of commentaries, one of which was coauthored by chemistry professor Daniel G. Nocera of Massachusetts Institute of Technology (Proc. Natl. Acad. Sci. USA 2006, 103, 15729; Science 2007, 315, 798).
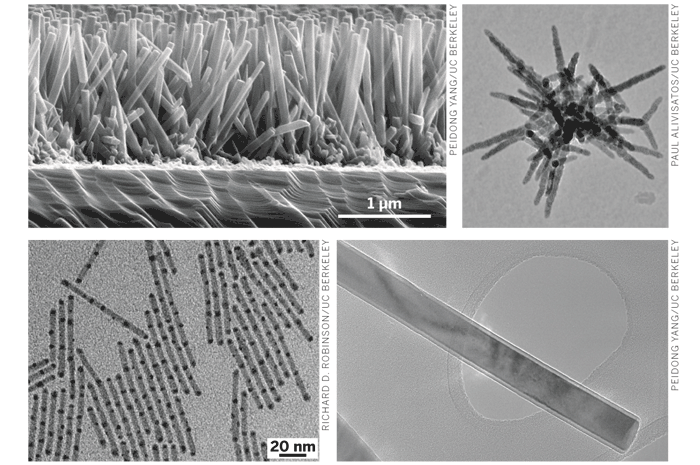
Many laboratories are looking beyond silicon toward next-generation photovoltaic materials. At the University of California, Berkeley, for example, chemist A. Paul Alivisatos and his colleagues are exploring the potential of semiconductor nanocrystals such as cadmium selenide and cadmium telluride. In recent years, the group has developed synthesis methods for tailoring the morphology, optoelectronic characteristics, and other properties of inorganic nanocrystals. Now they're putting those skills to use to make new types of experimental solar cells.
One of the reasons Alivisatos and coworkers focus on inorganic nanocrystals is that those materials can be processed via readily scalable colloidal chemistry techniques, which opens the door to a host of low-cost manufacturing possibilities. Similar to organic materials, the inorganic colloids can be deposited by spin-casting from solution onto a rigid support, where they spontaneously form the active light-sensitive layer in a solar cell. Or the tiny crystals can be processed as films on flexible sheets.
Compared with typical high-temperature and high-vacuum vapor-deposition methods used for fabricating silicon solar cells, solution-phase procedures can offer significant cost savings. Furthermore, according to Alivisatos, the low-tech and low-cost liquid-phase methods are well-suited to forming nearly defect-free nanosized crystals, which are endowed with unique physical properties that can be exploited to boost solar-cell performance.
A few years ago, Alivisatos' group blended rod-shaped nanocrystals of CdSe with the semiconducting conjugated polymer poly(3-hexylthiophene) (P3HT) to make hybrid inorganic-organic solar cells. They made the devices by sandwiching a 200-nm-thick layer of the blended photovoltaic material between an aluminum electrode and a transparent conducting electrode, which was deposited on an indium tin oxide glass support.
Overall, the power conversion efficiency, the effectiveness with which the device converts solar photons into electricity, was rather low—only around 2%. In contrast, common commercial silicon solar cells nowadays have 12 to 18% efficiencies, and top-of-the-line silicon devices can operate near 25%. Yet the UC Berkeley study demonstrated the ease with which the inexpensive materials could be fashioned into a working power generator and provided a test case from which researchers can learn about device performance.
One property critical to the performance of all solar cells is the ability of the photovoltaic layer to transport charge carriers. In the hybrid nanorod-polymer cells, CdSe serves as the transport material for electrons and P3HT as the transport medium for holes (positive charges that are the result of the absence of electrons). In a conventional solar cell, both of those functions are mediated by the silicon crystal.
In silicon devices, photons impinging on the semiconductor layer excite electrons from the material's valence band to its conduction band (across the band gap), thereby generating electron-hole pairs. To enhance the mobility of charge carriers in silicon, the semiconductor is generally doped with impurity atoms such that the material contains a positively charged (p-type) region and negatively charged (n-type) region. In that way, electron-hole pairs created near the interface between the two types of regions (known as a p-n junction) will be affected by a potential difference across the interface. Electrons will migrate toward the positive side of the junction while holes will rush toward the negative side, leading to a flow of electric current.
Unlike crystalline silicon solar cells, which feature a well-demarcated p-n junction, the cells under study by Alivisatos' team, and now by many other research groups, have numerous nanoscale junctions or interfaces between p-type and n-type materials. Distributed junctions, so named because of the large number of interfaces that are distributed throughout the light-sensitive layer, can enhance the charge-transport process. But they can also hinder it.
"Going down the nano route can be risky," Alivisatos warns. Replacing bulk semiconductors with nanostructured materials leads to solar cells with a lot of internal surfaces that can trap charge carriers and rob the device of its would-be flow of electric current. To avoid those problems, Alivisatos and coworkers have been trying to come up with useful strategies—"tricks" as he calls them—most of which involve chemical methods for manipulating and controlling matter on the nanometer scale.
WHILE STUDYING the CdSe nanorod-polymer systems, for example, the researchers found that elongating the nanocrystals, which they do synthetically, improves solar-cell performance. They observed that nanorods work better than round nanodots and that longer rods work better than shorter rods due to the decrease in the number of interfaces. So they began searching for ways to prepare colloidal crystals with a variety of rodlike morphologies.
The UC Berkeley group soon developed reproducible methods for making CdTe tetrapods (four-armed structures) and other kinds of semiconductor nanocrystals that feature rodlike arms and branches. That work led to their discovery of multiarmed inorganic dendrimers and then three-dimensional hyperbranched crystals.
The group reasoned that the extensive network of arms in the hyperbranched structures might boost solar-cell output relative to nanorod-based devices by providing a contiguous pathway for electrons to be transported through the photovoltaic layer to the solar cell's anode. It turns out that the multiarmed crystals do work better-but only slightly (about 2.5% power efficiency compared with 2% for cells made with single-rod nanocrystals). Yet, according to Alivisatos, they require far less effort to prepare than the nanorod solar cells (Nano Lett. 2007, 7, 409).
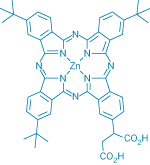
|
To dye for Zinc phthalocyanines are
used in dye-sensitized solar cells to harvest red and near-infrared
radiation efficiently.
By combining two types of semiconductors, the team has made solar cells that register as much as 3% power efficiency. By pairing materials that differ by just the right amount in the energy levels of their valence and conduction bands, in this case CdTe and CdSe nanorods, Alivisatos' group demonstrated that they could eliminate the organic polymer from the hybrid devices and make robust, all-inorganic solar cells using low-tech wet chemistry methods (Science 2005, 310, 462). And just last month, the team reported a new way to pair up semiconductors. Using a simple partial ion-exchange procedure, the group can now embed Ag2S nanodots in CdS nanorods. Alivisatos notes that this latest trick, which has not yet been tested in a solar cell, is applicable to many materials and offers a selective way to tune optoelectronic properties of inorganic nanocrystals (Science 2007, 317, 355). |
Down the hall at UC Berkeley, Peidong Yang is another chemist who designs experimental solar cells using nanostructured materials. His approach to sidestepping the charge-trapping problem is to use nanowires as direct electrical pathways for quickly transporting photogenerated electrons through a solar cell and collecting them as electrical current. Two years ago, after devising a synthetic method for growing arrays of ZnO nanowires in aqueous solution, Yang, graduate students Matt Law and Lori E. Greene, and their coworkers demonstrated the nanowire solar-cell idea in a proof-of-concept study (Nat. Mater. 2005, 4, 455).
Yang's nanowire photovoltaic work built upon the dye-sensitized solar-cell design pioneered by Michael Grätzel, a chemistry professor and director of the Laboratory of Photonics & Interfaces at the Swiss Federal Institute of Technology, Lausanne. Commonly referred to as Grätzel cells, those devices feature a network of disordered and inexpensive TiO2 nanoparticles that are coated with light-harvesting dye molecules and are typically surrounded by a liquid-phase electrolyte. Photons captured by the dye-generally a ruthenium complex-generate electron-hole pairs that separate at the surface of the nanoparticles. Electrons are injected and transported through the TiO2 layer while positive charges migrate via the electrolyte to the opposite side of the cell.
| "The problem with nanoparticle solar cells," Yang explains, "is that electrons have to hop randomly from particle to particle through the semiconductor layer until they reach the electrode. It's like trying to get from San Francisco to San Jose by following city streets. Any imperfection in the material acts as a roadblock, and the electrons get stuck." Yang's thinking was that dye-coated nanowires that span from one of the cell's electrodes to the other would enable electrons to flow through the devices in an unobstructed manner. "It would be like traveling on a highway instead of a city street," he says. |

Mitch Jacoby/C&EN charge maker Efros explains how a single photon can generate multiple electron-hole pairs in nanocrystals. |
In the 2005 study, the group showed that ZnO nanowire solar cells do indeed outperform ZnO nanoparticle cells by a factor of roughly 100 in terms of electron diffusivity, which is related to electron mobility. On average, the cells proved to be approximately 1.5% efficient. Since then, the group has improved the average efficiency further—to nearly 3%—by coating the ZnO nanowires with a shell of TiO2 (J. Phys. Chem. B 2006, 110, 22652). The core-shell structure provides a barrier that inhibits recombination of electrons and holes, Yang explains. He adds that the work is at an early stage and there's plenty of room for optimization.
While Yang has been developing strategies for incorporating nanowires into dye-sensitized solar cells, Grätzel and coworkers in Lausanne continue searching for other ways to improve those devices. Several years ago, Grätzel's team demonstrated that by using ruthenium polypyridyl dye complexes, their solar cells could operate at 10 to 11% efficiency. Yet, as Grätzel explains, that high level of performance requires using a relatively thick layer of TiO2 (more than 15 µm) as well as volatile electrolyte-solvent mixtures that are difficult to contain inside a solar cell, especially at elevated temperatures.
TO ADDRESS those issues, Grätzel, Shaik M. Zakeeruddin, and their coworkers have been pursuing the use of nonvolatile electrolytes such as ionic liquids. They also have synthesized novel dyes with enhanced light sensitivity, which decreases the requisite thickness of the dye-coated TiO2 layer. One of their promising new dyes, dubbed K-19, is similar to one of their older top-performing ruthenium dyes (labeled N3) but differs in a key way: One of N3's bipyridyl-dicarboxylate groups has been replaced by a styryl-substituted bipyridine. That change leads to a compound that is a stronger light absorber, and the researchers say it also should enhance solar-cell longevity (J. Am. Chem. Soc. 2006, 128, 4146).
Another drawback of the ruthenium family of sensitizers is their weak response to the red portion of the solar spectrum. One class of compounds that absorbs intensely in that region is phthalocyanines. But researchers have had little success in finding suitable members of that family for use in dye-sensitized solar cells for a number of reasons, including a mismatch in the electronic levels of the TiO2 particles and the dye molecules they support.
Now, Grätzel, Paidi Yella Reddy, Mohammad K. Nazeeruddin, and their coworkers have synthesized and tested a novel sensitizer compound that has performed well in initial tests: an unsymmetrical zinc phthalocyanine that contains three tert-butyl groups and two carboxylic acid groups (Angew. Chem. Int. Ed. 2007, 46, 373). The researchers note that phthalocyanine-sensitized solar cells may be useful as photovoltaic windows that transmit a portion of the sun's radiation in the visible wavelength region, while harvesting light efficiently in the red and near-infrared part of the spectrum. Not only would such windows generate electricity, the group points out, they would also cut down on the need for air-conditioning by reducing solar heating in the building.
| Organic polymers are another class of materials that investigators
around the globe are looking into for use in low-cost photovoltaic
devices. Similar to dye-sensitized solar cells and cells made from
nanocrystals, "plastic" solar cells based on blends of semiconducting
polymers and fullerene derivatives also transport charge by way of
numerous nanoscale interfaces between positive- and
negative-charge-carrying media (polymers and fullerenes, respectively).
And just like their low-cost inorganic cousins, polymer solar cells are
the subject of a steady flow of high-profile journal articles. Just within the past few weeks, researchers have reported on polymer solar cells that feature advanced designs and new types of polymers and ones that are made with new processing methods. In Hong Kong, for example, scientists from several institutions teamed up to synthesize a new low-band-gap polymer following predictions that such a material would be effective in polymer photovoltaics. That work led to a new platinum metallopolyyne-fullerene cell that exhibited an efficiency of more than 4% (Nat. Mater. 2007, 6, 521). At the University of California, Santa Barbara, researchers doubled the efficiency of solar cells made from a polydithiophene-fullerene mixture by adding a small amount of a processing agent to the solution from which the cells are spin cast. On the basis of photovoltaic measurements and atomic force microscopy, chemistry Nobel Laureate Alan J. Heeger, Guillermo C. Bazan, and their colleagues discovered that alkanedithiols of suitable chain length can alter the film's morphology and boost its power efficiency from 2.8% to 5.5% (Nat. Mater. 2007, 6, 497). |

Alivisatos

Lewis

Grätzel
Mitch Jacoby/C&EN (all)
|
IN RELATED work, Heeger, Kwanghee Lee, and their coworkers demonstrated that tandem polymer solar cells—a design in which two cells are connected in series—can be fabricated entirely using inexpensive solution-phase methods. Connecting two cells made from semiconductors with distinct band gaps broadens the range of wavelengths the system can collect to generate electricity. In contrast to other researchers who have employed high-vacuum deposition methods to prepare tandem cells, the UC Santa Barbara team fabricated their stacked devices from two polymer-fullerene blends and an intervening transparent titanium oxide layer-all three of which were processed and deposited from solution (Science 2007, 317, 222).
Somewhere between dye-sensitized solar cells and polymer-fullerene cells are polymer-sensitized solar cells. John R. Reynolds, Kirk S. Schanze, and their coworkers at the University of Florida have synthesized a new family of ionic organic polymers known as hyperbranched conjugated polyelectrolytes by way of a condensation reaction that joins thiophene and triphenylamine vinylene units. The team reports that self-assembling cationic-anionic bilayers of the material hold promise as a novel type of sensitizer due to their adhesion and light-gathering properties (J. Am. Chem. Soc. 2007, 129, 8958).
Experimentalists aren't alone in the search for new ways to design efficient, yet low-cost, photovoltaic devices. Theoreticians are working on that problem, too. One topic under theoretical investigation deals with capturing photons that "waste" their energy by heating solar cells instead of generating electricity. Typically, when photons in the blue region of the spectrum are absorbed by a solar cell, their energy, which exceeds the semiconductor's band gap, causes inefficient excitations that are converted to heat. But sometimes those photons generate multiple electron-hole pairs (also known as excitons).
If scientists could understand the mechanism that underlies multiexciton formation, they might be able to design new types of solar cells that get more photovoltaic bang for their buck-that is, two or three times as much electrical current for every high-energy photon absorbed.
At the Naval Research Laboratory in Washington, D.C., theoretician Alexander L. Efros has teamed up with Arthur J. Nozik of the National Renewable Energy Laboratory, in Golden, Colo., and other scientists and developed a theoretical explanation for that phenomenon in nanocrystals of PbSe, PbS, and CdSe (Nano Lett. 2006, 6, 2856). A number of experimentalists have already reported observing the multiexciton effect. Now scientists are devising ways of testing the new theory and putting the effect to practical use.
It's tough to predict which class of materials and solar-cell design will be the winning combination that generates a supply of clean, renewable, and affordable energy that is plentiful enough to make a significant contribution to the world's future needs. The solution may come from new types of devices and novel materials yet to be discovered or from creative ways of using substances already in hand. For now, the search goes on, and the sun continues to shower the planet with 120,000 TW.
Copyright © 2007 American Chemical Society To subscribe or visit go to: http://pubs.acs.org