What is a hydrogen battery?
A hydrogen battery is a combination of a fuel cell power module and
an energy module based on Hydrogen on Demand® technology.
Like a traditional battery, a hydrogen battery converts chemical energy into electrical energy. However, there are important differences.
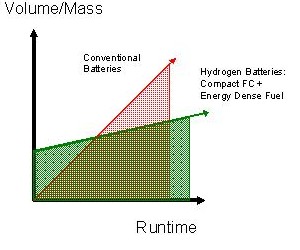
Traditional batteries are sealed systems. The volume of the battery limits the amount of energy the device can supply, which is in turn limited by the amount of active “fuel” material the battery contains. Once that fuel is consumed, the entire battery must either be replaced or recharged.
In a hydrogen battery the amount of energy the device can supply is not limited by the volume of the device. Because the hydrogen battery has separate power and energy modules, the hydrogen battery can be refueled and used over and over simply by replacing the energy module (refueling the system). The energy module can be more compact and less expensive than a primary, single use, battery and can be replaced in a moment, instead of recharged for an hour or more.
Advantages for hydrogen batteries vs. traditional batteries:
| • | The amount of energy possible is easily variable. For a traditional battery to supply energy for a longer time, the size of the entire sealed battery system must increase. A hydrogen battery system sized for a particular power can supply energy as long as it is refueled, keeping the size of the power module constant. |
| • | Because the fuel for a hydrogen battery packs more energy per unit volume and weight (energy density) than traditional batteries, the size of a hydrogen battery energy supply increases less with runtime than does a traditional battery. |
| • | The cost of obtaining additional energy from a hydrogen battery is simply the cost of the fuel. The cost of additional energy when using traditional primary batteries is that of purchasing more batteries, which can be many times the cost of the fuel. |
| • | A hydrogen battery can be “recharged” virtually instantaneously by supplying additional fuel; traditional battery recharging can take considerably longer, and is impossible in the absence of an auxiliary source of electrical power. |
Inside the Millennium Cell Hydrogen Battery – Power and Energy
Power
The power module half of the hydrogen battery is a fuel cell. Of existing
fuel cell technologies, the proton exchange membrane fuel cell (PEMFC, or
sometimes just PEM) has received significant attention for portable
electronic device applications. These fuel cells operate at low temperature
(< 80 °C) and use a solid polymer electrolyte. PEM fuel cells have a number
of advantages vs. alternative technologies that make them particularly
suitable for use in a hydrogen battery.
Unlike direct methanol fuels cells (DMFC), which operate on methanol and water and use a complicated process that requires more platinum at the electrodes, PEM systems use a simpler process requiring less costly electrodes. In addition, PEMs are more efficient, deliver higher voltages, and are more compact than a DMFC system of equivalent power.
Energy
The energy half of a hydrogen battery is, as one might expect,
hydrogen. Hydrogen is an ideal fuel for fuel cells because the conversion of
hydrogen and oxygen to energy is simple and uncomplicated by side reactions.
The only byproduct of a hydrogen fuel cell is water, which typically
vanishes into the atmosphere as harmless water vapor. The key practical
issue is storing enough hydrogen in the hydrogen battery for it to be
useful.
There are a several options for storing hydrogen for use in hydrogen batteries, but many have important draw-backs:
| • | Compressed hydrogen—the object of great attention for transportation, cannot supply enough fuel from a sufficiently small and light package for a portable device. |
| • | Metal hydrides, which absorb hydrogen on charging and release it on use are too heavy for portable use; they also require a local source of hydrogen for reuse and are too expensive to discard after only one use. |
| • | Because PEM fuel cells require hydration (wetting) of the membrane, both compressed hydrogen and hydrogen from a metal hydride source require humidification subsystems. |
| • | Hydrogen from methanol or other hydrocarbons, formed via a “reformation” process, requires high internal temperatures, complicated fuel processors, slow start-ups and results in carbon dioxide emissions. Purification is also usually required for use on PEM fuel cells. |
These systems are light, do not require purifiers, complicated fuel processors or high temperatures; they start quickly and do not emit greenhouse gasses.