Iron-air batteries audition for grid storage role
Researchers at the University of Southern Calif.'s Loker Hydrocarbon Research Institute say they have come up with iron electrodes that could make iron-air batteries practical for storing energy as generated by intermittent renewable sources such as wind and solar.
But don't expect to see iron-air batteries in hybrid vehicles or EVs. The energy density of iron-air batteries, even USC's version of them, is several times less than that of conventional lithium-ion units.
The claim to fame of USC's work is that the resulting cells can be made with materials that are relatively easy to come by and will last through several thousand high-rate charge/discharge cycles. The application USC researchers envision for them is in temporary energy storage at wind and solar installations where battery weight and size isn't much of an issue.
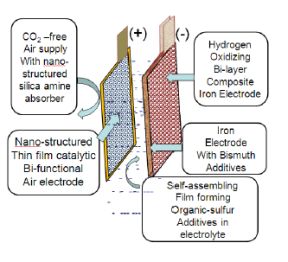
USC researchers placed chemical additives on the battery’s iron-based electrode and restructured the catalysts at the molecular level on the battery’s air-based electrode. These measures help the battery resist degradation and boost its life span.
Key to the battery performance is the development of electrodes prepared from super-pure carbonyl iron powder consisting of spherical iron particles (3 to 5-micron diameter) treated to remove any residual oxygen and carbon. The electrode surface gets infused with elemental bismuth which suppresses the evolution of hydrogen during charging, a problem in conventional iron-air cells because it uses up electrolyte. USC researchers prepare electrodes by combining the iron active material with a polyethylene binder material and pressing it into shape, followed by the application of heat.
They say such electrodes are inexpensive to fabricate (though the super-pure iron material itself can be a bit pricey). Earlier iron-air batteries tended to use sintering processes to make electrodes, which entailed high-temperature treatment in an inert gas, a process more expensive than simple pressing.
The resulting electrodes give a charge-discharge characteristic that has an efficiency in the 90% range, meaning most of the energy put into the battery gets stored, and vice versa.
All in all, the USC researchers saw a ten-fold reduction in the rate of hydrogen evolution and a discharge capacity of 0.3 A-hr/gm, compared to previous iron-air chemistry, and also a twenty-fold increase in capacity for the two-hour discharge rate. They published a paper describing their results in the Journal of the Electrochemical Society: http://jes.ecsdl.org/content/159/8/A1209.full
Reprints and Licensing
© 2012 Penton Media Inc. http://eetweb.com