Scientists develop catalyst that cleans diesel emissions without platinum
By David Szondy
August 17, 2012

Nanostellar has developed a mineral catalyst that outperforms platinum at a fraction of the cost (Photo: Shutterstock)
Diesel engines are a classic example of good news and bad news. The good news is that diesel engines are much more fuel efficient than petrol engines. The bad news is that they belch out some pretty nasty emissions like nitric oxide and nitrogen dioxide. The good news is that catalytic converters can scrub those out. The bad news is that last Friday the platinum needed by the converters is selling for US$1,473.10 an ounce. Now the good news is that a team at Nanostellar in Redwood, California, has developed a mineral catalyst that outperforms platinum at a fraction of the cost.
Platinum is an excellent catalyst, though it does have a few problems. One of the biggest at the moment is that a violent labor dispute in South Africa sent the price skyrocketing. Also, with the World Health Organization classifying diesel exhaust as a carcinogen, the potential demand for platinum for catalytic converters for hundred of millions of vehicles far outstrips supply. The Nanostellar team, led by Dr. Kyeongjae “K.J.” Cho, professor of materials science and engineering and physics at UT Dallas and co-founder of Nanostellar, determined that a mineral catalyst would be a cheaper alternative.
Dr. Kyeongjiae "K.J." Cho, professor of materials science and engineering and physics at UT Dallas (Photo: University of Texas at Dallas)
Reporting their findings in the August 17 issue of Science, Cho relates that computer modelling showed that mullite was a cost-effective substitute. Mullite is a silicate mineral discovered on the Isle of Mull, Scotland in 1924. It’s rare in nature, but a synthetic version is produced commercially for use in various porcelains, such as crucibles and heating balls. It has a very high melting point of 1840 C (3344 F) and as a mixed-phase oxide mineral it makes a very attractive catalyst. In addition, laboratory tests indicate that converters using mullite would have 45 percent lower emissions than with platinum.
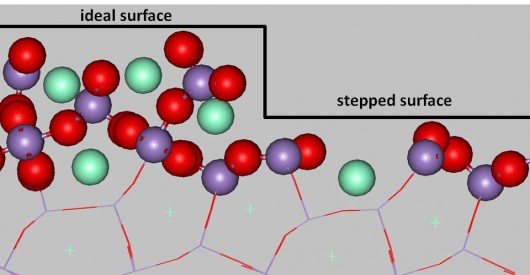
Ideal and stepped crystal surface of Noxicat (Image: Geoffrey McCool)
“Our goal to move completely away from precious metals and replace them with oxides that can be seen commonly in the environment has been achieved,” Dr. Cho said. “We’ve found new possibilities to create renewable, clean energy technology by designing new functional materials without being limited by the supply of precious metals.”
The new catalyst, called Noxicat, will be developed for commercial use and further work is planned to determine its application in fuel cells.
Source: University of Texas at Dallas via Phys.org
Copyright © gizmag 2003 - 2012 To subscribe or visit go to: http://www.gizmag.com