Air + water = gasoline? Not quite...
By Brian Dodson
October 30, 2012

Is there a future for synthetic gasoline? (Photo: Shutterstock)
According to Allen Ginsberg's poetic rewording of the laws of thermodynamics:
-
1. You can't win
-
2. You can't break even
-
3. You can't quit
Air Fuel Synthesis, Ltd. (AFS), a small company in the northern English county of Durham, has recently made headlines for a chemical process that claims to synthesize gasoline from air and water. In essence, AFS is using energy to unburn fuel so that it can be burned as fuel again – a great deal of energy. Sixty kWh of electric energy are used up to store 9 kWh of that energy in a liter of gasoline. When you take into consideration that gasoline vehicles are about 15 percent efficient, a car fueled with synthetic gasoline would use roughly 35 times more energy on a given trip than would an electric vehicle. Not, it would seem, a prescription for a commercially valuable green product.
What is gasoline? Not a single compound, gasoline is largely made of a mixture of saturated hydrocarbons, which are carbon skeletons combined chemically with about two hydrogen atoms per carbon atom. The right mixture of saturated hydrocarbons will burn quietly (if not cleanly) in a standard car's engine.
The history of making synthetic gasoline from other natural materials stretches back almost a century, with the first coal liquefaction plant going into service in Germany in 1919.
Coal liquefaction has remained a niche market, although there are oil-poor areas (e.g. South Africa) where a large fraction of liquid fuels are produced in this manner. But coal liquefaction has a number of annoying technological difficulties, as well as one very large problem for the future of our planet – synthetic gasoline made using coal liquefaction is not a carbon-neutral source of power. Instead, it takes carbon which was removed from the ecosphere in eons past and releases it in the form of carbon dioxide.
The Air Fuel Synthesis, Ltd. synthetic gasoline pilot plant in Northern England
Rather than mine carbon from the Earth in the form of coal, AFS acquires its carbon from the CO2 in the atmosphere. The result is a process whose only net production of CO2 is related to the power required to drive synthesis of the fuel. The general approach has been suggested by numerous people over the years, but AFS appears to be the first commercial company to work out the details and put together a pilot plant. Admittedly, the pilot plant is only producing about 1 percent of the projected yield per day, but the technological problems can be solved, as they have been in other contexts.
Let's break down the process into the individual steps.
The AFS gasoline synthesis process
-
The AFS synthetic gasoline process
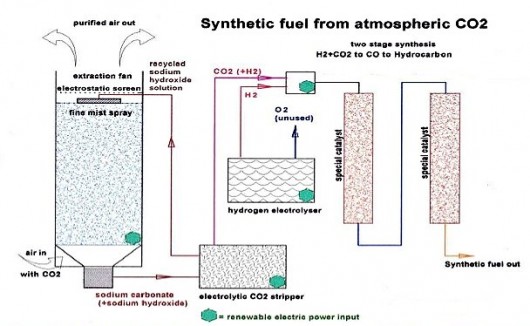
- Remove carbon dioxide from air by blowing air through a mist of sodium hydroxide solution, converting CO2 to sodium carbonate;
- Remove CO2 from sodium carbonate by electrolysis;
- Make hydrogen gas from water by electrolysis;
- Convert the carbon dioxide into carbon monoxide using the reverse water gas shift reaction;
- Convert hydrogen and carbon monoxide into gasoline using the Fischer-Tropsch process.
Again, all of these steps depend on well-known chemical reactions. Indeed, a very closely related process for collecting and converting CO2 and water into hydrocarbon fuels was patented by the US government in 2008.
The remaining issues are not primarily technical, but will be driven instead by economics and public policy. In a world where power supplies are vetted for their global warming damage, nearly all stationary energy users will be better served by some energy source other than liquid hydrocarbon fuels, particularly with a 15 percent energy storage ratio for synthetic gasoline. (The energy storage ratio is the amount of energy generated by burning a fuel divided by the amount of energy required to produce that fuel.)
In the AFS and similar processes, this is made worse in that the highest "grade" of energy (electric power) is being converted into a source of low-grade heat energy. In automobiles, for example, the efficiency for conversion of gasoline's heat energy into motive power is in the neighborhood of 15 percent, compared to an efficiency of about 80 percent for an electric car. This means a synthetic-gasoline-powered car will require about 35 times more electric power from a utility source for a given trip than will an electric car – hard to justify for an upcoming period in which our primary energy supplies will be severely tested. In addition, synthetic gasoline shares the other emissions problems of fossil fuels, particularly including CO2 and nitrogen oxide generation.
To synthesize sufficient gasoline to supply the world's present consumption would require about half of all energy currently being used by civilization in any form – heating, manufacturing, lighting, transportation, and so on. At present, it's estimated that gasoline consumption represents about 6.5 percent of total world energy consumption. Everything considered, it is hard to imagine any situation in which synthetic gasoline can compete with electric power for transportation. To be sure, there are other needs for portable power in which the large energy density of gasoline may be beneficial, but the net sum of these probably still points to what at best will be a minor niche market for synthetic fuels.
Source: Air Fuel Synthesis
About the AuthorCopyright © gizmag 2003 - 2012 To subscribe or visit go to: http://www.gizmag.com