Earth Warming Effect Only Due to CFC Destruction of Stratospheric Ozone!
A greater than normal warming occurred from 1966 until 2002 but
no measurements confirm an increase in CO2 emissions,
whether anthropogenic or natural, had any effect on global
temperatures. As a matter of fact, all atmospheric gases and dust in
our atmosphere cools our planet, they doesn't warm it[1].
There is very strong evidence that anthropogenic emissions of
chlorofluorocarbons (CFCs) were the only cause of the near recent
abnormal warming. CFCs were used primarily in air conditioning
units. Acting in accordance with an international treaty called the
Montreal Protocol (1987); the U.S. Environmental Protection Agency
(EPA) mandated the phase-out of CFCs (R-22) through the Clean Air
Act.
CFCs and other halides created both unnatural atmospheric cooling
and warming based on these facts. CFCs destroyed ozone in the lower
stratosphere-upper troposphere causing these zones in the atmosphere
to cool 1.37 o C from 1966 to 1998. The ozone loss
allowed more UV light to pass through the stratosphere at a
sufficient rate to warm the lower troposphere plus 10" of the earth
by 0.48 o C (1966 to 1998) [2]. The effect of
banning CFC production started having its effect around 2002. Since
2002 there has been no warming, see Figure 1.
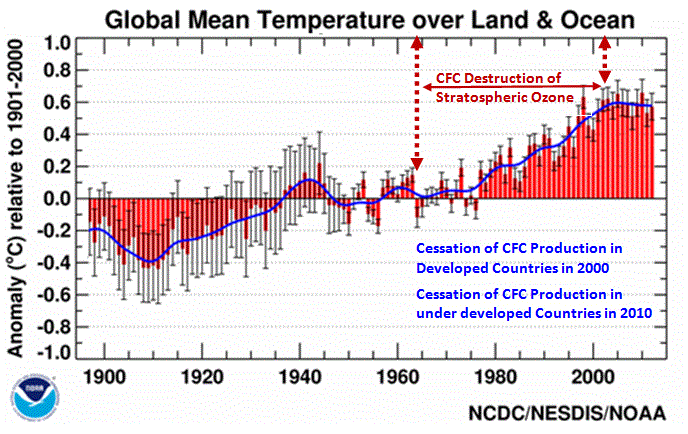
Figure 1. Global Mean Temperature, 1901 to 2010[3].
Stratospheric ozone was diminished by CFCs and other
refrigerants-propellants released into the atmosphere. These
compounds are broken down by the sun's UV rays and release chlorine
and bromine molecules that destroy the ozone. Scientists estimate
that one chlorine atom can destroy 100,000 ozone molecules over its
life in the stratosphere. With less ozone in the stratosphere, more
UV rays hit earth, warming it up and increasing the risk of skin
cancer.
The ozone layer extends from 8 km (upper troposphere) up throughout
the stratosphere. It is well known that the warming of the
stratosphere is caused by the reaction of ultraviolet light with
ozone. Energy is absorbed and ozone (O3) converts to
diatomic (O2) and (O) nascent oxygen. Conversely, ozone
loss decreases the amount of UV light absorbed and thus causes the
stratosphere to cool.
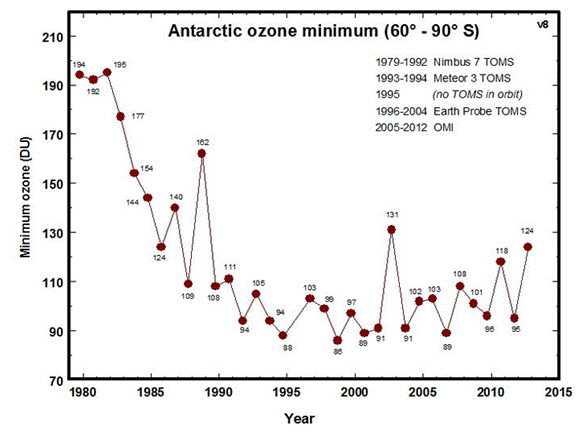
Figure 2. Antarctic ozone over time.
Figure 2 shows the lowest
value of ozone measured by TOMS (Total Ozone Mapping Spectrometer)
each year [4], a satellite instrument used to determine
ozone levels. CFCs, chlorinated solvents, halons, methyl bromide,
methyl chloride and Halogenated Chlorofluorocarbons (HCFCs) in the
stratosphere have begun a slow decline after reaching a peak in the
mid 1990s. The slow reduction is the result of the Montreal Protocol
of 1987 and later amendments. CFC production ceased in developed
countries in 2000 and stopped in underdeveloped countries in 2010[5].
The decline is now about 1% per year and the ozone is also now
increasing slightly in the stratosphere.
By around 2100 the ozone should be back to the levels seen in 1980.
Ozone in the year 2002 was higher in the ozone hole because of
unusually high temperatures in the Antarctic stratosphere (probably
due to more interaction with air outside of the Antarctic region).
The global average ozone is about 300 Dobson units. Before 1980
ozone less than 200 Dobson units was rarely seen. In recent years
ozone near 100 Dobson units has become normal in the ozone hole. The
Dobson unit is the most common unit for measuring ozone
concentration. One Dobson unit is the number of molecules of ozone
that would be required to create a layer of pure ozone 0.01
millimeters thick at the surface of the earth at a temperature of 0
degrees Celsius and a pressure of 1 atmosphere.
The legendary hypotheses of Paul Crutzen, Mario Molina, and Sherwood
Rowland, and led to CFCs being banned because they were destroying
stratospheric ozone Total stratospheric organic chlorine is
currently over 2.5 ppbv, see Figure 3.
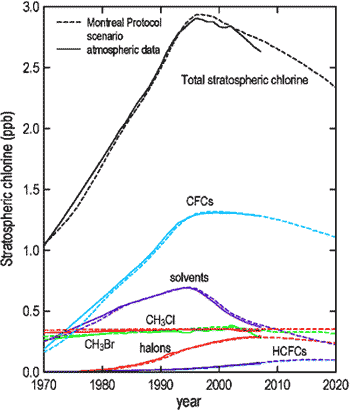
Figure 3. Stratospheric Chlorine[6].
One can see how the reduction
in stratospheric chlorine has affected global temperature. As it
stopped its rise in 1998 and started decreasing around 2002 the
temperature also started decreasing slightly (refer back to Figure
1).
Conclusions
Since 1966 it is apparent that CFC destruction of stratospheric
ozone was the only mechanism that caused the earth to warm. Since
2000 when CFC production was stopped in developed countries and CFC
concentration in the stratosphere stopped increasing, the earth
temperature has not increased.
Recently[7], Qing-Bin Lu of the University of Waterloo
stated, "a new theoretical calculation on the greenhouse effect of
halogenated gases shows that they (mainly CFCs) could alone result
in the global surface temperature rise of ~0.6°C from 1970-2002.
These results provide solid evidence that recent global warming was
indeed caused by the greenhouse effect of anthropogenic halogenated
gases".
The author is pleased that someone else has determined that CFCs not
CO2 and has caused the earth to warm. The author
discovered the CFC effect back in 2009[2], Dr. Lu has
been touting this for years as well but most scientists haven't
accepted it. Well, in the author's opinion as well as Dr. Lu's they
are wrong.
References:
1. “Gas Molecules and Dust in the Atmosphere Cool the Earth!”, Ashworth, Jan 19,2012,
http://www.energypulse.net/centers/article/article_display.cfm?a_id=2500
2. “CFC Destruction Major Cause of Recent Global Warming!”, Hydrocarbon Processing articles, October and
November publications, 2009. http://www.energypulse.net/centers/article/article_display.cfm?a_id=2152
3. Global Surface Temperatures Anomalies, National Oceanic and Atmospheric Administration, National Climatic
Data Center, Sept. 17, 2012. http://www.ncdc.noaa.gov/cmb-faq/anomalies.php
4. "The Ozone Hole Tour: Part II. Recent Ozone Depletion". Centre for Atmospheric Science, Cambridge
University, UK. March 28, 2011.
5. Nobel Prize in Chemistry, The Royal Swedish Academy of Sciences, October 11, 1995.
http://nobelprize.org/nobel_prizes/chemistry/laureates/1995/press.html
6. CSIRO Atmospheric Research and Cape Grim Baseline Air Pollution Station, the Australian Antarctic
Division and Australian Bureau of Meteorology.
http://www.environment.gov.au/soe/2006/publications/drs/indicator/14/index.html
7. Qing.-Bin Lu, International Journal of Modern Physics B, “Cosmic-Ray-Driven Reaction and Greenhouse
Effect of Halogenated Molecules: Culprits for Atmospheric Ozone Depletion and Global Climate Change”,
DOI: 10.1142/S0217979213500732, May 30, 2013.
![]()
Copyright © 2002-2013, CyberTech, Inc. - All rights reserved .http://www.energypulse.net
http://www.energycentral.com/generationstorage/environmentalemissionsandcarbonmanagement/articles/2682
