Ocean Denitrification
Denitrification is a microbially facilitated process of nitrate reduction that may ultimately produce molecular nitrogen (N2) through a series of intermediate gaseous nitrogen oxide products. In general, it occurs where oxygen, a more energetically favorable electron acceptor, is depleted, and bacteria respire nitrate as a substitute terminal electron acceptor. Denitrification only takes place in anoxic environments where oxygen consumption exceeds the oxygen supply and where sufficient quantities of nitrate are present. As ice sheets melted during the deglaciation of the last ice age and global oceans warmed, oceanic oxygen levels decreased and denitrification accelerated by 30 to 120 percent, a new international study shows, creating oxygen-poor marine regions and throwing the oceanic nitrogen cycle off balance. By the end of the deglaciation, however, the oceans had adjusted to their new warmer state and the nitrogen cycle had stabilized — though it took several millennia. Recent increases in global warming, thought to be caused by human activities, are raising concerns that denitrification may adversely affect marine environments over the next few hundred years, with potentially significant effects on ocean food webs.
Results of the new study have been published in the journal Nature Geoscience.
"The warming that occurred during deglaciation some 20,000 to 10,000 years ago led to a reduction of oxygen gas dissolved in sea water and more denitrification, or removal of nitrogen nutrients from the ocean," explained Andreas Schmittner, an Oregon State University oceanographer and author on the Nature Geoscience paper. "Since nitrogen nutrients are needed by algae to grow, this affects phytoplankton growth and productivity, and may also affect atmospheric carbon dioxide concentrations."
"This study shows just what happened in the past, and suggests that decreases in oceanic oxygen that will likely take place under future global warming scenarios could mean more denitrification and fewer nutrients available for phytoplankton," Schmittner added.
In their study, the scientists analyzed more than 2,300 seafloor core samples, and created 76 time series of nitrogen isotopes in those sediments spanning the past 30,000 years. They discovered that during the last glacial maximum, the Earth’s nitrogen cycle was at a near steady state. In other words, the amount of nitrogen nutrients added to the oceans — known as nitrogen fixation — was sufficient to compensate for the amount lost by denitrification.
A lack of nitrogen can essentially starve a marine ecosystem by not providing enough nutrients. Conversely, too much nitrogen can create an excess of plant growth that eventually decays and uses up the oxygen dissolved in sea water, suffocating fish and other marine organisms.
Following the period of enhanced denitrification and nitrogen loss during deglaciation, the world’s oceans slowly moved back toward a state of near stabilization. But there are signs that recent rates of global warming may be pushing the nitrogen cycle out of balance.
"Measurements show that oxygen is already decreasing in the ocean," Schmittner said “The changes we saw during deglaciation of the last ice age happened over thousands of years. But current warming trends are happening at a much faster rate than in the past, which almost certainly will cause oceanic changes to occur more rapidly.
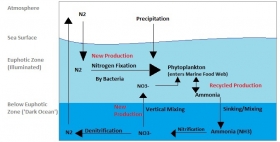
There is a marine nitrogen cycle. Nitrogen enters the water through precipitation, runoff, or as N2 from the atmosphere. Nitrogen cannot be utilized by phytoplankton as N2 so it must undergo nitrogen fixation which is performed predominately by cyanobacteria. Ammonia and urea are then released into the water by excretion from plankton. Nitrogen sources are removed from the euphotic zone by the downward movement of the organic matter. The sinking results in ammonia being introduced at lower depths below the euphotic zone. Bacteria are able to convert ammonia to nitrite and nitrate but they are inhibited by light so this must occur below the euphotic zone. Nitrification can then occur to convert the ammonium to nitrite and nitrate. Nitrate can be returned to the euphotic zone by vertical mixing and upwelling where it can be taken up by phytoplankton to continue the cycle. N2 can also be returned to the atmosphere through denitrification. This is a complex process and may be easily affected by a number of unknown factors.
For further information see Denitrification.
Nitrogen Cycle image via Wikipedia.
2013©. Copyright Environmental News Network