FDA Violates Free Speech to Limit Supplement Access
Did you know that if a supplement company “Likes” a customer’s Facebook post, it magically transforms nutritional supplements into drugs? The same thing happens if a supplement’s website links to a scientific article!
As recently as this week, the FDA has made attacks like these to
expand the agency’s definition of “disease claim.” Why? By
saying a supplement makes a disease claim, the FDA can call it a
“drug”—and then remove it from the market!
This also has scary implications for the government regulation of Internet searches and speech. If supplement companies start deleting customer comments out of fear of FDA action, then the FDA has effectively limited consumer speech by using the company as a censorship puppet.
It doesn’t stop there: the FDA has something called its Evidence-Based Review System. It’s just a backdoor tactic to require prohibitively expensive studies—as in hundreds of millions of dollars—if companies want to make any claim about the science behind their supplements. Because natural products can’t be patented, supplement companies can never recoup these astronomical costs. Only Big Pharma and their blockbuster drugs can afford to “pay-to-play:”
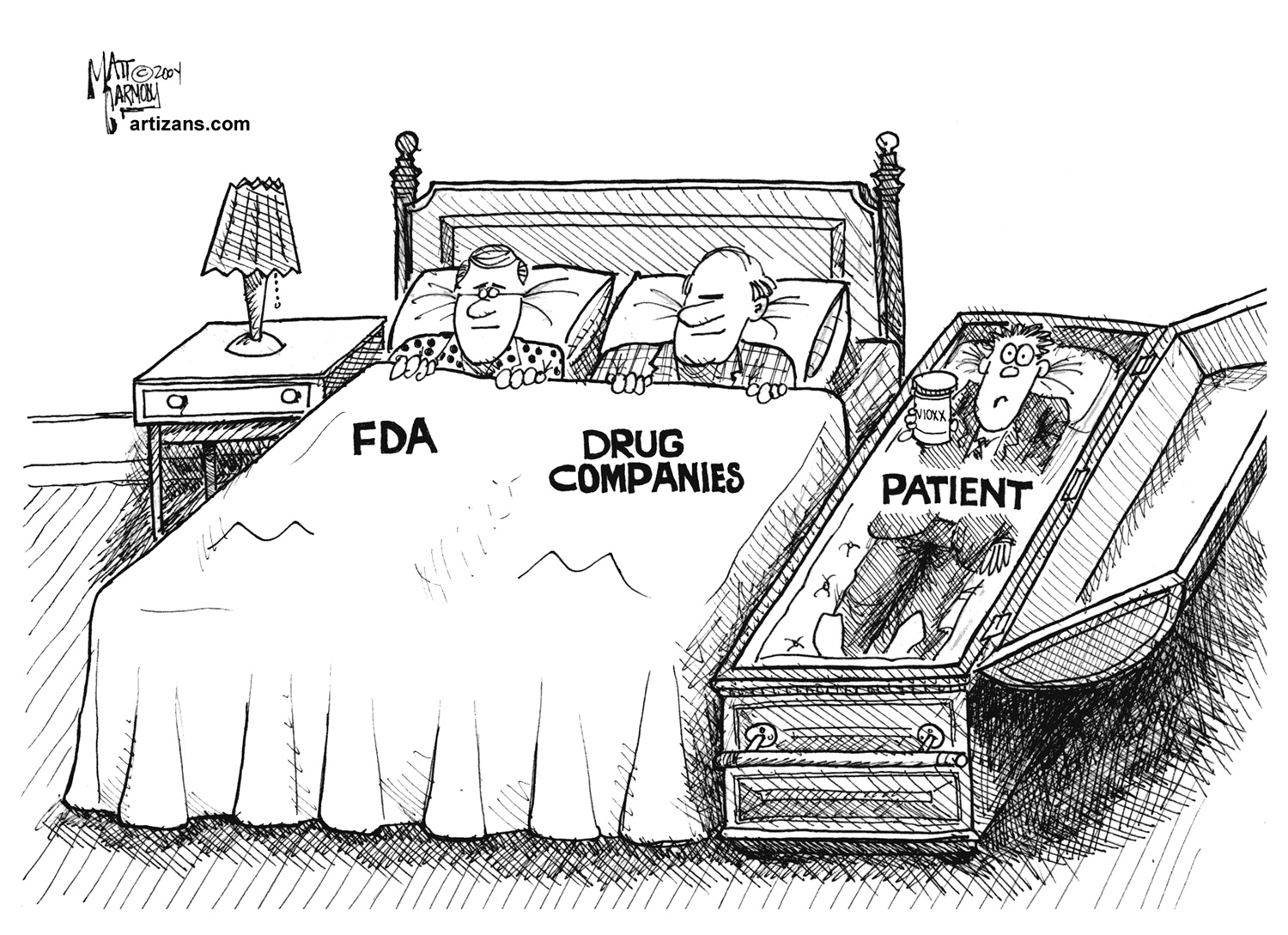
We’ve had enough. How about you?
Here’s our plan: ANH-USA will submit multiple petitions—strategically supported by our ongoing legal activities—to address, from various angles, FDA’s egregious First Amendment violations. If we win, we ALL win. If the petitions are denied, we’re ready to pursue further litigation.
Immediate action is critical. Unfortunately, this is an expensive project. This is why we need your help!
The Alliance for Natural Health USA
1350 Connecticut Ave NW, 5th Floor, Washington, DC 20036
Ph: 800.230.2762
www.anh-usa.org