by
Jacques Dufour
CNAM Laboratoire des sciences nucléaires, 2 rue Conté 75003
Paris France
Direct Download
Abstract:
The nuclear signatures that can be expected when contacting
hydrogen with nickel, were derived from thermal results
recently obtained (Rossi energy amplifier), using the type
of reaction paths proposed as the explanation of the energy
produced. The consequences of proton or neutron capture have
been studied. It was shown that these consequences are not
in line with the experimental observations. A novel
tentative explanation is thus described. Should this
explanation be true, it is proposed to call pico-chemistry
the novel field thus opened.
…
Introduction:
In a recent paper [1], it was shown that, if the reaction
path occurring in a Rossi energy amplifier [2], was mainly
proton capture, the lead thickness required to completely
suppress the gamma flux produced, would be in the order of
tens of cm. The lead screen used (2 cm) should thus have
resulted in a lethal gamma dose emitted in the surroundings.
Another explanation, different from proton or neutron
capture is thus to be found. In [3], the concept of
pico-chemistry was presented, that could explain the
generation of photons in the range of tens of keV, thus
compatible with the lead screening used in the energy
amplifier.
In chemistry, compounds are formed by the binding of the
components through their outer electronic shells. Ionic,
metallic and covalent hydrides of metals are known. Thus,
Nickel hydride NiH can be viewed as an hydrogen and a nickel
atoms maintained at a few angstrom distance, through a
metallic bound.
In contrast, in a pico-nickel hydride, a (shrunken) hydrogen
atom would be inside the electronic cortege of the Nickel
and bound to the nickel at close proximity of its nucleus.
In [3] a tentative explanation was given, of the possibility
of such an exotic hydride. Another approach is presented in
this paper.
Possible
existence of a small hydrogen-like dipole and reaction with
a nickel nucleus:
Various concepts of a shrunken hydrogen atom have been
presented. In [4], the possibility of having bound states of
a proton and an electron with lower radius and higher
ionization energy than the usual Bohr values is claimed.
These bound states were called hydrinos and attributed to
the possibility of having fractional values for the main
quantum number of the hydrogen atom. In [5] a metastable
state is justified by the electron spin/proton nuclear spin
interaction being first order in the environment of a
lattice (it is third order in vacuum). This state was called
hydrex and proposed as an explanation for fission-like
reaction occurring in metallic lattices. Finally, the
interaction of a proton and an electron could result in a
virtual neutron [6], that could be captured by and react
with the Ni nucleus.
In this paper, the evolution of a virtual neutron like
association between a proton and an electron, in contact
with an atom is examined.
At the surface of various materials (metals, metal oxides,
metal hydrides…), electrons are more or less free to leave
the solid (work function). In an hydrogen environment, it is
conceivable that from time to time a virtual neutron can be
formed between such an electron and a proton [6], with a
deficit of energy of 0.781 MeV:
The life time of this virtual neutron is limited by the
Heisenberg uncertainty relation ∆t∆E>h, which sets the
maximum distance d it can travel:
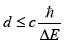
The maximum of d is thus some 250 fm and the virtual
neutron, formed at the periphery of an atom has hardly any
chance to reach the close vicinity of the nucleus of this
atom. It can nevertheless sufficiently penetrate the outer
electronic cortege of the atom so as to feel the (screened)
positive potential of the nucleus of the atom, when
reverting to a proton and an electron. An electrical dipole
is thus formed, which is attracted by the nucleus of the
atom. One can wonder if the resulting effect of the action
of the positive charge of the nucleus will ultimately end up
in the destruction of the dipole, the proton being rejected
to infinite and the electron bound to the nucleus. This
would certainly be the case if the nucleus where not
surrounded by its electronic cortege (Z time ionized
nucleus). In the case of an atom with its electrons, an
equilibrium position of the dipole could be reached, at
close vicinity of the nucleus. To demonstrate the
possibility of such a bound state, the complete Hamiltonian
of the system would have to be solved, which is not
possible. A semi-empirical approach has thus been developed,
to reach the orders of magnitude of the characteristics of
such a dipole and its interactions with an atom A. This
could be used as a guide when looking at the experimental
results expected in case of an excess energy measured in the
system hydrogen/nickel (energy of radiations emitted,
characteristics of the by-products).
In order to distinguish this concept of shrunken hydrogen
atom from others, it is proposed to call it Hypole
(Deupole and Tripole being
the 2 other isotopes).
Semi
empiric description of the Hypole:
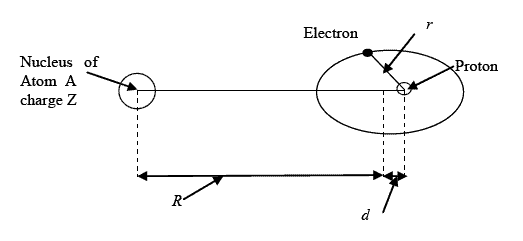
Figure 1 gives a description of the Hypole,
which is proposed to be written H¯Ni when the host
atom A is Nickel and its (possible) bound state with the
Ni atom, a Nickel pico-hydride NiH¯Ni.
d is the distance between the centers of gravity of
positive and negative charges in the hypole.
r is the distance between the proton and the
electron.
R is the distance between the center of the nucleus
of the atom A and the center of gravity of the hypole.
Z is the charge number of the atom A
The potential that the dipole proton/electron feels from A
is at first order (when d/R is small):
During its attraction by A, the spatial extension of the
dipole is limited by the repulsion of the inner layers of
the electrons of A, resulting in a shrinking of this
hydrogen-like object and in a limitation of its
polarization. In order to get first guesstimated values of
the size and energy of the hypole and of the bound state it
might form with A, following assumptions are made:
1. The action of the electronic cortege of A (especially the
s electrons of A) on the dipole and the presence at short
distance of the Z protons of A are equivalent to the
attraction of the electron by the proton in the hypole being
multiplied by a factor K>1. Hence, the (pseudo) coulomb
interaction in the dipole is:
2. d is small and proportional to R. Hence, d=kR,
with k small.
3. The electron of the hypole H¯A cannot be found
in the nucleus of the atom A (competition with the s
electrons of A). Hence, r≤R
With these assumptions, the Bohr radius of H¯A
would be:
and its energy of formation:
In a similar way, the Bohr radius of AH¯A would be:
and its energy of formation:
with mH being the mass of the hydrogen atom.
Under assumption 3, the smallest possible bound object AH¯A
is obtained for
In that case meK=mHKZ. Expressing the
energies as a function of the unknown k, one gets:
and
finally yielding the following value for the total energy
given by the hypole formation followed by its binding with
A:
The bulk of the energy is coming from the formation of the
Hypole. EH¯A likely to be of the order of magnitude
of the energies that can be found close to the A nucleus,
that is the s electrons energy E^sA.
A guesstimated value of k is thus:
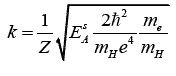
In the case of Nickel and taking for E^sNi the
average value 10.5 keV, the following guesstimated
description of H¯Ni and NiH¯ Ni is
obtained (Table 1):
Properties of the Hypole:
The hypole is a picometer size hydrogen-like
object. It can only exist when embedded in the electronic
cortege of an atom A, where its equilibrium position is very
close to the nucleus of A. Its size and energy of formation
depends upon A. In the case of Nickel, the size is some 2
picometer and the energy of formation round 10 keV. Hence
the names and notations proposed.
The best way for characterizing an hypole, is to measure
the mass of the corresponding A/pico-hydride. In the case of
nickel, following masses are expected, that take into
account the energy of formation (Table 2):

The mass differences given by Table 2
could be easily detected using a high resolution TOF Mass
Spectrometer on an acidic solution of the nickel
pico-hydride (probably possible see below, chemical
properties). SIMS TOF Mass Spectrometry is not adapted,
since the primary ions energies are of the order of the
energy of formation of the hypole. An ICP TOF Mass
Spectrometer would be adapted.
As regards the chemical properties of NiH¯Ni,
they should be close to the Nickel ones. The outer
electronic layers of NiH¯Ni indeed see the
positive charge of the nickel atom, the effect of the
hypole H¯Ni being second order in that respect.
A shift of the characteristic rays given by nickel in
ICP-AOS could be observed.
Finally the radiations emitted during the hypole
formation, would be photons in the 10 keV range, thus
completely suppressed by the 2 cm layer of lead in the
energy amplifier. Faint signals of higher energy photons
(annihilation radiation) could anyhow be detected. They
might be the signature of an inherent instability of the
hypole and of the corresponding pico-hydride, which is
discussed now.
Stability of the (nickel) hypole:
The nickel hypole is a small object of
picometer dimension and at picometer distance from the
nickel nucleus. Its virtual neutron state may have a non
zero probability to penetrate the nickel nucleus and react
with it according to the neutron capture route developed in
[6] and [1]. Most of the gamma photons resulting from the
stabilization of the primary excited nickel nuclei are of
energy higher than 1 MeV [1]. They mainly interact with the
lead shield by producing electron/positron pairs, ultimately
yielding the annihilation radiation. From the experimental
observations, the rate of virtual neutron capture should be
very low (some 10^-20 s^-1, in the experiment 2009(3-5/4-26)
presented in [2]).
Conclusions:
In this paper, a rough description is given,
of a novel chemical interaction. Orders of magnitudes of the
main characteristics of this still hypothetical interaction
are given.
It is hoped that this approach will be of help when
trying to understand the thermal results obtained with
the energy amplifier.
Should the experimental results and their interpretation
be true, pico-chemistry would become a reality.
References:
[1] J. Dufour “Nuclear signatures to be
expected from Rossi energy amplifier” Journal of nuclear
physics May 6th 2010
[2] S. Focardi and A. Rossi “A new energy source from
nuclear fusion” Journal of nuclear physics
[3] J. Dufour “Very sizeable increase of gravity at
pico-meter distance: a novel working hypothesis to explain
anomalous heat effects and apparent transmutations in
certain metal hydrogen systems” J. of condensed matter
nuclear science 1 (2007) p 47-61.
[4] R.L. Mills and W.R. Good “A unified theory derived from
first principles” Black light power, Inc. (1992)
[5] J. Dufour, D. Murat, X. Dufour and J. Foos “Experimental
observation of nuclear reactions in palladium and uranium:
possible explanation by the hydrex mode” Fusion Science and
Technology Vol.40-July 2001- p.91-106
[6] L. Daddi “Virtual neutrons in orbital capture” Journal
of nuclear physics March 18, 2010
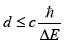
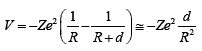
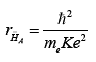
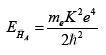
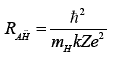
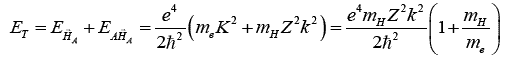
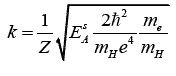


Dear Michele Bartoli:
I agree upon one fact: if we arrived here the merit has to be given to our Lord.
Warm Regards,
A.R.
La strada per scoprire e comprendere dove conduce lo studio sull’infinitamente piccolo che crea il tutto-esistente,probabilmente porta direttamente al cuore del Creatore; cuore del Creatore che agli umani non potrà mai essere permesso di sondare e penetrare in pieno; ma all’uomo allo stato attuale delle cose, un aiuto tramite qualcosa di indispensabile, come l’amplificatore di energia di Rossi, il Creatore amorevolmente lo ha concesso.
Adesso gli uomini di scienza, hanno la responsabilità di collaborare a far si che questo dono miracoloso, possa trasformarsi in utile realtà per l’intero pianeta.
[...] Is the Rossi energy amplifier the first pico-chemical reactor …Jul 18, 2010 … Is the Rossi energy amplifier the first pico-chemical reactor? by. Jacques Dufour CNAM Laboratoire des sciences nucléaires, 2 rue Conté … [...]
As I said many times, this reaction is based on Randell Mills “hydrinos”, or “shrunken hydrogen atoms” as you may call them.
What is needed to make “shrunken hydrogen”?- elevated temperature and certain “catalyst”.This was all openly disclosed by Mills in his publications and patents.In 1991 Thermacore filled patent for this kind of reaction.
So, what we need to have to start reaction?
1.Atomic hydrogen.To make this hydrogen gas H2 need to be heated and contacted with certain metals like Wolfram or Nickel-then it dissociates to atomic hydrogen.
2.Introduce atomic hydrogen at elevated teperature to certain “catalyst” , energy emission takes place when atomic hydrogen “shrinks” when reacts with catalyst substance. Catalysts were openly disclosed by Mills. One of them could be K2CO3.
3.”Shrunken” hydrogen atom then further reacts with Nickel metal, coming into crystalline structure of Ni more easily- then propably neutron/proton capture mechanism takes place yelding more energy.
So, as we see, energy is created during at least 2 steps: “shrinking” of atomic hydrogen ( Mills theory) and then by neutron/proton capture of “shrunken” hydrogen atom with Ni metal.
Fact, that nanometer Ni powder is in use multiplies reaction area and probability of reaction.
Summarising,the “father” of this reaction is Randell Mills.Without his “shrunken” hydrino atom reaction of neutron/proton capture could not take place.
Of course there are also other important things- like Ni powder preparation. Ni powder should be super-clean and de-gassed in deep vacuum to remove all impurities.
If I would design a cell- I would use inside reactor Ni powder with one of Mills catalysts in powder state.Then by use of el. heater inside introduce H2 gas.
All those reaction steps will take place inside reactor. At elevated temperature and presence of Ni powder H2 gas will dissociate to nascent hydrogen, then will “shrunk” in contact with “catalyst” and in “shrunken” state penetrate more easily into Ni metallic structure causing further reactions.
Regards,
pix
Copyright © 2014 Journal of Nuclear Physics - All Rights Reserved
http://www.journal-of-nuclear-physics.com/?p=275