
1. A pilot manufacturing plant at 24M’s headquarters in Massachusetts has produced thousands of test batteries to demonstrate the efficiency of the new design. (Image courtesy of 24M).
An advanced manufacturing approach for lithium-ion batteries, developed by researchers at MIT and at a spinoff company called 24M, promises to significantly slash the cost of the most widely used type of rechargeable batteries while also improving their performance and making them easier to recycle.
“We’ve reinvented the process,” said Yet-Ming Chiang, the Kyocera Professor of Ceramics at MIT and a co-founder of 24M (and previously a co-founder of battery company A123). The existing process for manufacturing lithium-ion batteries, he said, has hardly changed in the two decades since the technology was invented, and is inefficient, with more steps and components than are really needed.
The new process is based on a concept developed five years ago by Chiang and colleagues including W. Craig Carter, the POSCO Professor of Materials Science and Engineering. In this so-called “flow battery,” the electrodes are suspensions of tiny particles carried by a liquid and pumped through various compartments of the battery.
The new battery design is a hybrid between flow batteries and conventional solid ones: In this version, while the electrode material does not flow, it is composed of a similar semisolid, colloidal suspension of particles. Chiang and Carter refer to this as a “semisolid battery.”
Simpler Manufacturing Process
This approach greatly simplifies manufacturing, and also makes batteries that are flexible and resistant to damage, says Chiang, who is senior author of a paper in the Journal of Power Sources analyzing the tradeoffs involved in choosing between solid and flow-type batteries, depending on their particular applications and chemical components.
This analysis demonstrates that while a flow-battery system is appropriate for battery chemistries with a low energy density (those that can only store a limited amount of energy for a given weight), for high-energy-density devices such as lithium-ion batteries, the extra complexity and components of a flow system would add unnecessary extra cost.
Almost immediately after publishing the earlier research on the flow battery, Chiang said, “We realized that a better way to make use of this flowable electrode technology was to reinvent the [lithium ion] manufacturing process.”
Instead of the standard method of applying liquid coatings to a roll of backing material, and then having to wait for that material to dry before it can move to the next manufacturing step, the new process keeps the electrode material in a liquid state and requires no drying stage at all. Using fewer, thicker electrodes, the system reduces the conventional battery architecture’s number of distinct layers, as well as the amount of nonfunctional material in the structure, by 80 percent.
Having the electrode in the form of tiny suspended particles instead of consolidated slabs greatly reduces the path length for charged particles as they move through the material—a property known as “tortuosity.” A less tortuous path makes it possible to use thicker electrodes, which, in turn, simplifies production and lowers cost.
Bendable and Foldable
In addition to streamlining manufacturing enough to cut battery costs by half, Chiang says, the new system produces a battery that is more flexible and resilient. While conventional lithium-ion batteries are composed of brittle electrodes that can crack under stress, the new formulation produces battery cells that can be bent, folded, or even penetrated by bullets without failing. This should improve both safety and durability, he says.
The company has so far made about 10,000 batteries on its prototype assembly lines, most of which are undergoing testing by three industrial partners, including an oil company in Thailand and Japanese heavy-equipment manufacturer IHI Corp. The process has received eight patents and has 75 additional patents under review; 24M has raised $50 million in financing from venture capital firms and a U.S. Department of Energy grant.
The company is initially focusing on grid-scale installations, used to help smooth out power loads and provide backup for renewable energy sources that produce intermittent output, such as wind and solar power. But Chiang says the technology is also well suited to applications where weight and volume are limited, such as in electric vehicles.
Another advantage of this approach, Chiang says, is that factories using the method can be scaled up by simply adding identical units. With traditional lithium-ion production, plants must be built at large scale from the beginning in order to keep down unit costs, so they require much larger initial capital expenditures. By 2020, Chiang estimates that 24M will be able to produce batteries for less than $100 per kilowatt-hour of capacity.
Venkat Viswanathan, an assistant professor of mechanical engineering at Carnegie Mellon University who was not involved in this work, said the analysis presented in the new paper “addresses a very important question of when is it better to build a flow battery versus a static model. … This paper will serve as a key tool for making design choices and go-no-go decisions.”
Viswanathan adds that 24M’s new battery design “could do the same sort of disruption to [lithium ion] batteries manufacturing as what mini-mills did to the integrated steel mills.”
In addition to Chiang, the Power Sources paper was co-authored by graduate student Brandon Hopkins, mechanical engineering professor Alexander Slocum, and Kyle Smith of the University of Illinois at Urbana-Champaign. The work was supported by the U.S. Department of Energy’s Center for Energy Storage Research, based at Argonne National Laboratory in Illinois.
Preventing Fires in Lithium Batteries
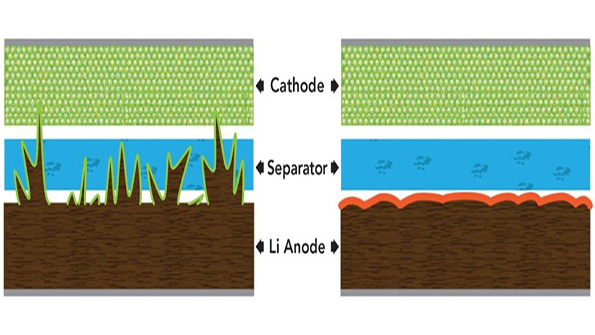
In a study that could improve the safety of next-generation batteries, researchers discovered that adding two chemicals to the electrolyte of a lithium metal battery prevents the formation of dendrites— “fingers” of lithium that pierce the barrier between the battery’s halves, causing it to short out, overheat and sometimes burst into flame.
The findings, published June 17 in Nature Communications, could help remove a major barrier to developing lithium-sulfur and lithium-air batteries, promising future technologies that could store up to 10 times more energy per weight than batteries now used in consumer electronics and electric cars.
“Because these batteries would be much lighter than today’s rechargeable batteries, they have a lot of potential for extended-range electric vehicles,” said Yi Cui, an associate professor at Stanford University and the Department of Energy’s SLAC National Accelerator Laboratory. “But one of the things that’s been holding them back is their tendency to form dendrites, which are also the culprit behind overheating and occasional fires in today’s lithium-ion batteries.”
A New Approach to Safety
Dendrites form when a battery electrode degrades, and metal ions become deposited on the electrode’s surface. When those finger-like deposits elongate until they penetrate the barrier between the two halves of the battery, they can cause electrical shorts, overheating, and fires.
In a previous study published last October, Cui and his colleagues reported that they had developed a “smart” lithium-ion battery that senses when dendrites start to puncture the barrier so the battery can be replaced before the situation becomes dangerous. This could offer a solution for millions of batteries now in use in cell phones, laptops, and other devices, as well as in electric cars and airplanes.
The new research addresses battery technologies that haven’t reached the market yet, and it takes a different approach: adding chemicals to the electrolyte to prevent dendrite formation. One compound, lithium nitrate, has been under investigation for a long time as an additive to improve battery performance. The other, lithium polysulfide, has been considered a nuisance: Formed when a sulfur electrode degrades, it travels to the lithium metal electrode and wrecks it, Cui said.
In brainstorming sessions, the research team realized their combined effect had not been studied before; together the chemicals could potentially react with lithium metal to form a stable, solid interface between the electrode and the electrolyte.
Improved Performance
They assembled coin-cell batteries, similar to the ones that power calculators, remote controls, and watches, and added various concentrations of the two chemicals to the ether-based electrolyte. Then they ran those batteries through many charge/discharge cycles, took them apart and examined the electrodes with an electron microscope and an X-ray technique that reveals their morphology and chemical composition.
They found that adding both chemicals in just the right amounts stopped lithium dendrite formation; harmless pancake-like deposits grew instead. The lithium metal electrode acquired a stable coating that helped protect it from further degradation and actually improved the battery’s performance (Fig. 2).
Harnessing Synergy
“This is a really exciting observation,” she said. “We had been doing experiments all along with these two chemicals in there, but this was the first time we looked at the synergistic effect. This does not completely solve all the problems associated with lithium metal batteries, but it’s an important step.”
Yet-Ming Chiang, the MIT professor whose new Li-ion battery manufacturing and design process is making waves, collaborated with the team and helped them interpret their results. He said the next step is to see if this approach can prevent dendrite formation in larger-scale cells that are closer to being practical batteries. It may also work for electrodes made of other metals, such as magnesium, calcium, or aluminum, that also have potential for storing much more energy than today’s batteries.
“Preventing dendrite formation is going to be key to their success,” Chiang said.
Funding for the project was provided by the Joint Center for Energy Storage Research (JCESR), a Department of Energy Innovation Hub, and Cui and Chiang are both JCESR principal investigators.