Scientists extract carbon dioxide from atmosphere and use it to make batteries
Could replace phone and electric car batteries and create a zero-emission solution

Excessive atmospheric carbon dioxide is reaching catastrophic
proportions, forcing national governments, industrial leaders,
and scientists to band together and come up with a solution. So
far, most talks have dealt reducing future carbon dioxide
emissions, but few have designs for reducing the carbon dioxide
already in the atmosphere. That is, until now.
An interdisciplinary team of scientists have found a way to replace the graphite electrodes in lithium-ion batteries with carbon electrodes sourced directly from the atmospheric carbon dioxide. The research has wide application in consumer electronics and more importantly, electric vehicles, potentially capable of creating an automobile that’s carbon negative, reducing the amount of CO2 in the atmosphere as they drive.
Leading the research was the laboratory of Cary Pint, assistant professor of mechanical engineering, Vanderbilt University, and the laboratory of Stuart Licht, professors of chemistry, George Washington University; their findings are described in the journal ACS Central Science.
According to professor Licht, the research aims to “transform the greenhouse gas carbon dioxide into valuable products and to provide greenhouse gas emission-free alternatives to today's industrial and transportation fossil fuel processes.”
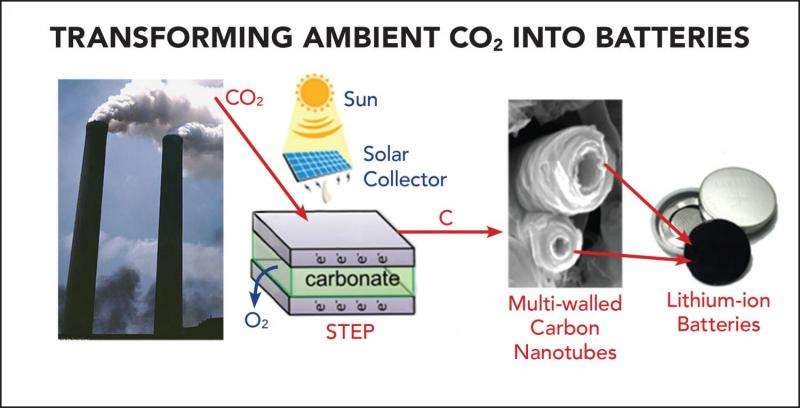
The team used the solar thermal electrochemical process (STEP)
to supply the appropriate amounts of electrical and thermal
energy needed to deconstruct carbon dioxide into carbon and
oxygen, which were then used to produce carbon nanotubes capable
of functioning as the positive electrode or anode in lithium-ion
batteries.
But it doesn’t end there; the nanotubes proved equally capable of fulfilling the role of the positive electrode in sodium-ion batteries, a low-cost solution being developed for the electric grid and other large-scale applications.
"This approach not only produces better batteries but it also establishes a value for carbon dioxide recovered from the atmosphere that is associated with the end-user battery cost unlike most efforts to reuse CO2 that are aimed at low-valued fuels, like methanol, that cannot justify the cost required to produce them," said Pint.
Batteries created from carbon nanotubes offer a significant performance boost over the graphite counterparts, with no decline in capacity after 2.5 months of continuous charging and discharging. The team noted that small defects in the carbon, a side effect of STEP, resulted in sodium-ion batteries exhibiting stable storage performance over 3.5 times above that of sodium-ion batteries with graphite electrodes.
Pint estimates that—depending on the specifications—40 percent of the battery could be made out of recycled carbon dioxide, not including the outer protective packaging. What’s more, with the production of lithium-ion batteries costing approximately $325 per kWh, a single kilo of carbon dioxide has an estimated value of $18 as a battery material—which is six times more than if it were converted to methanol—a figure that only increases when transitioning from large batteries used in EV, to smaller batteries used in electronic devices. In addition, unlike methanol, the merging of batteries with solar cells provides clean energy with zero greenhouse emissions.
But why stop there? Licht suggests that the solar thermal electrochemical process be coupled to natural gas-powered electrical generators to create a fossil fuel electrical power plant with zero net carbon dioxide emissions. For example, as a generator produces heat and electricity, it also creates a concentrated form of carbon dioxide, which would reinforces the STEP if fed back into the system. Oxygen, another byproduct of STEP, could then be channeled back into the generator, causing a boost in its combustion efficiency to compensate for the amount of electricity that STEP consumes.
Source: PHYS.orgviaVanderbilt University
Learn more about Electronic Products Magazine