
Copyright 1995 by the American Geophysical Union, 2000
Florida Ave., N.W., Washington, DC 20009. Permission is granted to journalists
to use material in this publication at their discretion and to individual
scientists for research or classroom use. For permission for other uses, contact
the AGU Publications Office.
Printed version ISBN number 0-87590-865-9

Upper tropospheric humidity patterns on April 27, 1995, at 1745 UTC as measured by the GOES 8 satellite. The 6.7 m data are related to water vapor, with a peak signal between about 550 and 350 mbar. The data are influenced by high clouds. (Image courtesy of A. Gruber and R. Achutuni, NOAA National Environmental Satellite Data and Information Service.)
The climate of Earth is able to support life in large part because of the atmospheric greenhouse effect and the workings of the hydrological cycle. Water in the gaseous phase, water vapor, is a key element in both of these. This report provides a basic description of the scientific understanding of the roles water vapor plays in the climate system.
The hydrological cycle describes the movement of water, in all three phases, within and between the Earth's atmosphere, oceans, and continents. In the vapor phase, water moves quickly through the atmosphere and redistributes energy associated with its evaporation and recondensation. The movement of water vapor through the hydrological cycle is strongly coupled to precipitation and soil moisture, which have important practical implications. The basic operation of the hydrologic cycle is well known, but some details are poorly understood, mainly because we do not have sufficiently good observations of water vapor.
There are many atmospheric greenhouse gases, some naturally occurring and some resulting from industrial activities, but probably the most important greenhouse gas is water vapor. Water vapor is involved in an important climate feedback loop. As the temperature of the Earth's surface and atmosphere increases, the atmosphere is able to hold more water vapor. The additional water vapor, acting as a greenhouse gas, absorbs energy that would otherwise escape to space and so causes further warming. This basic picture is complicated by important interactions between water vapor, clouds, atmospheric motion, and radiation from both the Sun and the Earth. There are some aspects of the role of water vapor as a greenhouse gas that are not well understood, again mainly because we lack the necessary observations to test theoretical models.
Monitoring long-term changes in water vapor, which are closely linked to other climate variations and trends, is needed to both predict and detect changes. This report describes traditional measurement systems and promising new technologies that together may provide the continuity and quality of observations needed to improve our understanding of water vapor in the climate system.
The report is based on the work of the AGU Chapman Conference on Water Vapor in the Climate System held October 25-28, 1994, at Jekyll Island, Georgia, but encompasses material from other sources and the thoughtful input of many scientists throughout its preparation and review.
Specific humidity is the ratio of the mass of water vapor in a sample to the total mass of the moist air, including both the dry air and the water vapor. The mixing ratio is the ratio of the mass of water vapor to the mass of only the dry air in the sample. As ratios of masses, both specific humidity and mixing ratio are dimensionless numbers. However, because atmospheric concentrations of water vapor tend to be at most only a few percent of the amount of air (and usually much lower), they are both often expressed in units of grams of water vapor per kilogram of (moist or dry) air. Absolute humidity is the same as the water vapor density, defined as the mass of water vapor divided by the volume of associated moist air and generally expressed in grams per cubic meter. The term is not much in use now.
The partial pressure of a given sample of moist air that is attributable to the water vapor is called the vapor pressure. The vapor pressure necessary to saturate the air is the saturation vapor pressure. Its value depends only on the temperature of the air. (The Clausius-Clapeyron equation gives the saturation vapor pressure over a flat surface of pure water as a function of temperature.) Saturation vapor pressure increases rapidly with temperature: the value at 90°F (32°C) is about double the value at 70°F (21°C). The saturation vapor pressure over a curved surface, such as a cloud droplet, is greater than that over a flat surface, and the saturation vapor pressure over pure water is greater than that over water with a dissolved solute.
Relative humidity is the ratio of the actual vapor pressure to the saturation vapor pressure at the air temperature, expressed as a percentage. Because of the temperature dependence of the saturation vapor pressure, for a given value of relative humidity, warm air has more water vapor than cooler air. The dew point temperature is the temperature the air would have if it were cooled, at constant pressure and water vapor content, until saturation (or condensation) occurred. The difference between the actual temperature and the dew point is called the dew point depression.
The wet-bulb temperature is the temperature an air parcel would have if it were cooled to saturation at constant pressure by evaporating water into the parcel. (The term comes from the operation of a psychrometer, a widely used instrument for measuring humidity, in which a pair of thermometers, one of which has a wetted piece of cotton on the bulb, is ventilated. The difference between the temperatures of the two thermometers is a measure of the humidity.) The wet-bulb temperature is the lowest air temperature that can be achieved by evaporation. At saturation, the wet-bulb, dew point, and air temperatures are all equal; otherwise the dew point temperature is less than the wet-bulb temperature, which is less than the air temperature.
In addition, water vapor is the most abundant of the greenhouse gases in the atmosphere and the most important in establishing the Earth's climate. Greenhouse gases allow much of the Sun's shortwave radiation to pass through them but absorb or trap the longwave, infrared radiation emitted by the Earth's surface. Without water vapor and other greenhouse gases in the air, surface air temperatures would be well below freezing.
The concentration of water vapor in the atmosphere reflects the number of molecules of water compared with the total number of air molecules (mainly nitrogen and oxygen). There are many ways to define the atmospheric water vapor concentration. In this text we will use mainly mixing ratio and relative humidity. Mixing ratio is the measure of the mass of water vapor in a kilogram of air. Relative humidity reflects the ratio of the actual pressure of water vapor in a sample of air to the pressure necessary to saturate that air at a given temperature.
Warm air can sustain a higher concentration of water vapor than cooler air without becoming saturated. Consequently, as air warms, for whatever reason, more evaporation may take place and the concentration of water vapor may increase. An increase in water vapor enhances the greenhouse effect and gives rise to further warming. This positive feedback, warming from increased greenhouse gases leading to an increase of water vapor and therefore even more warming, is a feature of climate models used for estimating the effect of increased greenhouse gases. According to the 1990 Intergovernmental Panel on Climate Change (IPCC) report, Climate Change: The IPCC Scientific Assessment [Houghton et al., 1990], this feedback could amplify the temperature change due to a doubling of carbon dioxide by some 60%. The IPCC update, scheduled for release in 1996, does not change this conclusion. Thus an understanding of the mechanisms distributing water vapor through the atmosphere and of water vapor's effects on atmospheric radiation and circulation is vital to estimating long-term changes in climate.
Recent theoretical and observational advances have put a new focus on water vapor in the climate system but also have raised new questions.
The weight of the atmosphere's water vapor contributes only about one quarter of one percent of the total sea level pressure of all the gases. If all the water vapor in the air at a particular time were to condense and fall as rain, it would amount to a depth of only about 2.5 cm. This is called precipitable water. Because water vapor is not evenly distributed globally, there would be about 5 cm near the equator and less than one tenth as much at the poles. The average precipitation over the globe is about 1 m annually, so there must be a rapid turnover of water in the air; the average water molecule spends about 9 days in the air before precipitating back to the surface.
This rapid turnover, combined with the variation of temperature with height and geography, causes water vapor to be distributed unevenly in the atmosphere, not only horizontally but vertically as well. Figure 1 shows the mean vertical distribution of temperature and the mixing ratio of water vapor in the atmosphere. The lower scale shows that water vapor decreases rapidly with height as the atmosphere gets colder. Nearly half the total water in the air is between sea level and about 1.5 km above sea level. Less than 5-6% of the water is above 5 km, and less than 1% is in the stratosphere, nominally above 12 km. Relative humidity (not shown) also tends to decrease with height, from an average value of about 60-80% at the surface to 20-40% at 300 mbar (9 km). Despite the small amount of water vapor in the upper troposphere (above about 5 km) and stratosphere, recent research has shown that upper tropospheric water vapor is very important to the climate.
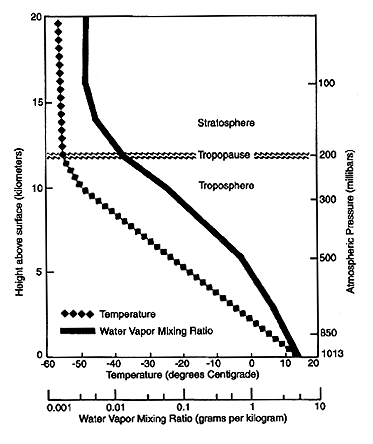
Figure 1. Schematic of the tropospheric and stratospheric layers and the tropopause, the boundary between the two. The mean vertical distribution of temperature and the water vapor mixing ratio in the atmosphere are shown. Note that the mixing ratio scale is logarithmic and the vertical scales give approximate conversions between atmospheric pressure (in millibars) and altitude (in kilometers). (Figure courtesy of Dian Gaffen, Air Resources Laboratory, Silver Spring, Maryland.)
The mean distribution of precipitable water, or total atmospheric water vapor above the Earth's surface, is shown in Figure 2. The general decrease of precipitable water from equator to the poles is a reflection of the global distribution of temperature. As expected, amounts of precipitable water are greatest over warm, equatorial regions and decrease more or less continuously with increasing latitude down to very low values over the cold, polar regions. There are exceptions in the major desert regions, where the surface air is very dry despite its high temperature. The most humid region is in the western equatorial Pacific, above the so-called "oceanic warm pool," where the highest sea surface temperatures are found.

Figure 2. The mean distribution of precipitable water, or total atmospheric water vapor above the Earth's surface, for 1992. This depiction includes data from both satellite and radiosonde observations. (Image courtesy of Thomas Vonder Haar and David Randel, Colorado State University, Fort Collins.)
Variations in the atmospheric water vapor field occur on timescales from a few minutes to decades. The pattern of water vapor changes with the seasonal changes in temperature and atmospheric circulation patterns. Seasonal variations appear to be stronger in the northern hemisphere than in the southern hemisphere, just as the corresponding temperature variations are stronger. The variations are stronger because of the northern hemisphere's larger fraction of land, which has a lower heat capacity than the ocean and thus responds more quickly to variations. On multiyear timescales, the large changes in the sea surface temperature in the tropical Pacific associated with the so-called El Niño-Southern Oscillation (ENSO) seem to cause shifts in the distribution of water vapor.
There have been several estimates of longer-term changes in tropospheric water vapor. The most recent global estimate shows an increase in precipitable water during the period 1973-1990, with the largest trends in the tropics, where increases as large as 13% per decade were found. A recent study of water vapor trends above North America based on radiosonde measurements from 1973 to 1993 finds increases in precipitable water over all regions except northern and eastern Canada, where it fell slightly. The regions of moisture increase are associated with regions of rising temperatures over the same period, and the regions of decreased moisture are associated with falling temperatures.
A unique program makes approximately monthly measurements of the vertical profile of water vapor with frost point hygrometers carried by balloons at Boulder, Colorado. Observations over a 14-year period (1981-1994) show an increase in water vapor in the lower stratosphere over Boulder of a little less than one 1% per year. Although it is not completely understood, the observed trend is not inconsistent with an observed increase in methane because methane oxidizes to water vapor in the stratosphere.
These recent results are intriguing, but it should be noted that the trends are observed over relatively short, recent periods and have limited spatial domains. Studies covering longer periods and other regions of the globe will require both the continuation of current measurement systems and improved observations in regions that are now poorly observed.
Water vapor is the link between the surface and the atmosphere in the water or hydrologic cycle. As is shown in Figure 3, almost all water vapor in the atmosphere originates at the surface of the Earth, where water evaporates from the ocean and the continents owing to the Sun's radiation, and is transpired by plants and respired by animals into the atmosphere. Once in the atmosphere, water vapor can be transported horizontally and vertically by the three-dimensional circulation of the atmosphere and may condense to form liquid water or ice crystals in clouds. The cycle is completed when water returns to the Earth's surface in various forms of precipitation such as rain or snow. This cycle is closely tied to atmospheric circulation and temperature patterns.

Figure 3. The global water or hydrologic cycle, showing estimates of contents of major reservoirs and rates of transfer or fluxes of water between them. (Image used by permission of the National Academy of Science [1987].)
The hydrologic cycle is strongly influenced by the nature of the surface of the Earth. Hydrological processes operate on different timescales over the ocean and land. Over the ocean, surface temperature, which varies slowly, is a major controlling factor, while over land, coupled effects of surface temperature and available soil moisture, which can change relatively quickly, are important.
Rivers carry water from land to oceans, from which we infer that there must be more precipitation than evaporation over land. To achieve balance, there must then be more evaporation than precipitation over oceans. The excess water vapor is transported from oceanic to continental areas and precipitates.
Water vapor transport is an important factor in the determination of global climate. Movement of water vapor in the atmosphere represents the movement of energy in the form of latent heat. Upon condensation, this latent energy is converted into sensible heat, or heat that can be felt, and thus represents a source of atmospheric heating. This condensation heating is a major source of energy for the circulation systems associated with weather and climate.
The effect of clouds on the climate system is complicated. Clouds reflect sunlight, which reduces solar radiation input to the Earth-atmosphere system. However, clouds also trap longwave radiation emitted by the Earth, as does water vapor. Clouds are highly interactive with the Earth's surface. They regulate the amount of sunlight received by the surface and so influence evaporation from the surface, which in turn influences cloud formation. Precipitation from clouds, in turn, influences soil moisture and evaporation rates. Soil moisture content and sunshine regulate the type of vegetation that covers the surface, which also influences evaporation rates. As the Earth's climate changes, we cannot predict whether the net effect of these interactive changes in cloudiness and other elements of the climate system will tend to amplify or reduce the change in climate.
The mechanics by which convective cloud systems transport water vapor vertically in the atmosphere are poorly understood. Cloud updrafts have long been thought to carry moisture to higher elevations, but evidence also suggests that cloud microphysical processes and cloud dynamics may dry the upper troposphere. However, computations with models making quantitative predictions conclude that cloud processes and large-scale water vapor transports increase upper troposphere water vapor. Large-scale storms, rather than small-scale convection, are believed to be the primary agent for moistening the upper troposphere. This is an area that needs much more study.

Figure 4. Some of the different types of ground- or space-based systems for observing water vapor.
It is generally agreed that improved knowledge of the role of water vapor in the climate system hinges largely on closing observational gaps that currently exist. To date, most large-scale water vapor climatological studies have relied primarily on analysis of radiosonde data, which have good resolution in the lower troposphere in populated regions but are of limited value at high altitude and are lacking over remote oceanic regions.
Recently, substantial progress has been made using satellite observations to obtain total column water vapor and some low-resolution vertical profiles from infrared and microwave sensors. Satellite observations do not provide water vapor data in all weather conditions above all surfaces. Special processing of signals received from the Global Positioning System (GPS), a satellite-based navigational tool, has been receiving increased attention recently as a method for measuring water vapor, as it could give long-term measurements of the total column water vapor. For more detailed local studies as well as measurements in the upper troposphere and lower stratosphere, Lyman alpha and frost point hygrometers (see Table 1) and differential absorption and Raman lidars can be useful.
New water vapor data sets have been constructed during the last several years from a combination of satellite remote-sensing methods and direct observations to achieve improved spatial coverage and vertical resolution. Figure 2 is a result of such an effort. Data assimilation systems, which combine information from observations and output from atmospheric models, also are being used to augment traditional observations and, in some instances, to take the place of data where no observations are available.
There are several efforts currently under way to observe, understand, and model the hydrological cycle and energy fluxes in the atmosphere, on the land surface, and in the upper ocean. The efforts will investigate variations of the global hydrological regime and their impact on atmospheric and oceanic dynamics. Variations in regional hydrological processes and water resources and their response to change in the environment such as the increase of greenhouse gases will be examined. The Global Energy and Water Cycle Experiment (GEWEX), a program launched by the World Climate Research Program (WCRP), will act as a coordinating body to facilitate some of these programs.
There are questions about how well the current models, both those used in climate studies and those used in forecasting the daily weather, treat water vapor. Modeling would be improved by systematic examination of models's treatment of water vapor in light of what is now known of its distributions. Some of the questions arise because of the lack of good water vapor observations. The likely benefits of improved water vapor data include better weather forecasts as well as improved climate models.
Different types of measurements are complementary and useful. The challenge is how best to merge the available information on water vapor distribution into an improved description of the time and space variations of water vapor to enhance climate studies.